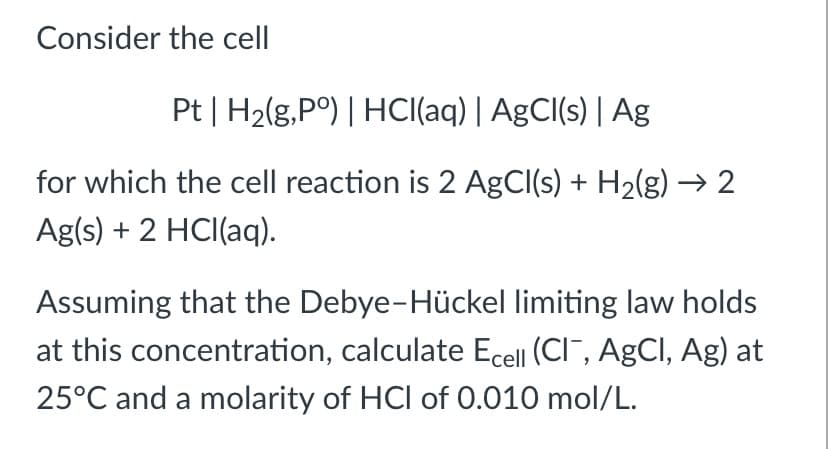
Pt | H2(g,P°) | HCI(aq) | AgCl(s) | Ag for which the cell reaction is 2 AgCI(s) + H2(g) → 2 Ag(s) + 2 HCI(aq). Assuming that the Debye-Hückel limiting law holds at this concentration, calculate Ecell (CI¯, AgCI, Ag) at 25°C and a molarity of HCI of 0.010 mol/L.
Q: Carbon may exist as diamond, graphite, and other allotropes. The standard Gibbs energy of formation…
A: Given: Carbon has different forms of allotropes such as diamond and graphite. The standard Gibbs…
Q: 4. The standard Gibbs energy of formation of PH3(g) is 13.4 kJ/mol at 298 K. Note that the standard…
A: Given: Partial pressure of PH3 = 2.0 bar Partial pressure of H2 = 2.0 bar Standard Gibbs free…
Q: The standard potential of the AgCl/Ag,Cl− couple fits the expressionE⦵/V = 0.23659 − 4.8564 ×…
A: standard Gibbs energy and enthalpy of formation of Cl−(aq) and its standard entropy are given below…
Q: The biological standard reaction Gibbs energy of the reaction of removing phosphate from adenosine…
A: Given, Energy is = -14 KJ/ mol. Temperature is = 298 K. pH is = 7. given, ∆G = ∆G° + R.TlnQ We have…
Q: Consider the decomposition of a metal oxide to its elements, where M represents a generic metal.…
A: .
Q: 11. An aqueous solution of aluminium chloride can be prepared by the redox reaction between…
A: (a) Aluminium is getting oxidized. (b) 2 mol of Aluminum gives = 3 mol of H2 0.0800 mol of…
Q: The standard reaction Gibbs energy of the decomposition of CaCO3 into CaO and CO2 at 1173 K is…
A: Given data is as follows: The standard Gibbs free energy of the reaction = +0.178 kJ/mol = 178 J/mol…
Q: Calculate the standard free-energy change at 298K for the zinc-copper voltaic cell, which has a…
A:
Q: How is equilibrium constant of a reaction related to standard cell potential?
A: How is equilibrium constant of a reaction related to standard cell potential?
Q: Under standard conditions, the electromotive force of the cell, Zn(s) | ZnCl₂(aq) | Cl₂(g) | Pt is…
A: Solutions Hence the standard entropy of the formation - 1.384 kj/mol
Q: The standard potential of the AgCl/Ag, CI couple fits the expression Eº/V = 0.23659-4.8564 x 10-4…
A:
Q: The biological standard potential of the couple pyruvic acid/lactic acid is -0.19 V at 25 °C. What…
A:
Q: Adenosine triphosphate, ATP, is used to store energy within biological systems. It releases that…
A: The reaction of ATP hydrolysis is shown below:
Q: Consider the cell Pt(s)/ H2(g, 1 bar)/NaOH (aq, 0.01m), NaCl (aq, 0.01125m)/ AgCl(s) / Ag(s) 2H₂O +…
A: Recall the cell representation, Pt s / H2 g, 1 bar / NaOH aq, 0.01 m,…
Q: how much does the cell potential change when Q is decreased by a factor of 10 for a reaction in…
A: Given , valence factro(v) = 2 T = 298 K Q is decreased by 10 Using Nernst's equation, E1 = E° -…
Q: The standard Gibbs energy of the reaction N2(g) + 3 H2(g) → 2 NH3(g) is −32.9 kJ mol−1 at 298 K.…
A:
Q: One enzyme-catalysed reaction in a biochemical cycle has an equilibrium constant that is 8.4 times…
A: K1 = 8.4 K2 ∆Gₒ1 = -2.303RTlogK1, ∆Gₒ1 = - 250 KJ/mol Standard reaction occur at 25ₒC or 298 K - 250…
Q: Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. 3…
A:
Q: For the reaction N2 (g) + 3H2(g) --> 2 NH3 (g), (a) what is the reaction Gibbs free energy at…
A: (a) For the given reaction, using values from the question and taking temperature in Kelvin scale,…
Q: Calculate the standard Gibbs energy of reaction for 4 HI(g) + O2(g) → 2 I2(s) + 2 H2O(l) at 298 K,…
A: Gibb’s free energy (∆G0)– For a reaction when ∆Gf0 product and ∆Gf0 reactant is known then,…
Q: Calculate the standard Gibbs energy change at 25 °C for the following reaction: Mg(s) +…
A:
Q: If we have a hydrophobic molecule in aqueous solution that makes contact with 25 water molecules,…
A: Hydrophobic molecules do not interact with the water molecule at normal temperature. Mixing of…
Q: The standard Gibbs energy for the hydrolysis of ATP to ADP is -31 kJ mol-. What is the Gibbs energy…
A: For the reaction., ATP=ADP+Pi ∆G=Go+RT lnQ Q = ([ADP/Pi]/(ATP]) ∆G=Go+RT ln ([ADP/Pi]/(ATP]) For…
Q: 5. For the cell Cd(Hg)|CdSO4 H2O(s)|Hg;SO4(s)|Hg the emf is given as a function of temperature t…
A: The emf as a function of temperature is given as, => E (V) = 1.01845 - 4.05 X 10-5 X (t - 20) -…
Q: The standard voltage, Eº, for the reaction of Zn(s) and Cl₂(g) is 2.12 V. What is the standard Gibbs…
A:
Q: The chemical potential of a particular substance in the region A of a system is 5kJ higher than…
A: Since the relationship between change in gibbs free energy and chemical potential is given by ΔG =…
Q: A fuel cell develops an electric potential from the chemical reaction between reagents supplied from…
A: (a) hydrogen and oxygen,
Q: Gibbs energy change, A:G®, is related to cell potential, E, by the equation Calculate the standard…
A: For the given cell, change in enthalpy and entropy at 25oC is as follows: ∆rHo = -729 kJ mol-1∆rSo =…
Q: By how much does the cell potential change (in volts) when Q is increased by a factor of 21 for a…
A: Answer:
Q: The standard potential of the AgCI/Ag,Cl- couple fits th expression E/V =…
A: Standard potential of the AgCl/Ag,Cl- couple as per the fit expression : Eo (V) = 0.23659 – 4.8564 x…
Q: The standard voltage, E°, for the reaction of Zn(s) and Cl,(g) is 2.12 V. What is the standard Gibbs…
A: Standard gibb's free energy is directly related to Standard EMF of the cell. Here ,Zn2++2e-→Zn and…
Q: Construct a cycle similar to that in Fig. 3D.3 to analyse the reaction 1/2 H2(g) + 1/2 I2(s) →…
A: The thermodynamic cycle for the reaction 1/2 H2(g) + 1/2 I2(s) → H+(aq) + I−(aq) hs to be drawn…
Q: In the smelting of iron ores, the reduction of iron oxides in a blast furnace is accompanied by the…
A: Gibbs free energy is a thermodynamic state function. It is a property of a system that measures the…
Q: In a research laboratory, the standard potential of the cell Pt(s)|H2(g)|HCl(aq)|AgCI(s)|Ag(s) was…
A:
Q: standard enthalpy
A:
Q: What is the mass-action expression, Qc, for the following chemical reaction? 4H30*(aq) + 2CI"(aq) +…
A:
Q: 3. Assuming standard states for all reactants and products, determine the spontaneous direction of…
A: The reactions given are, 1) Cu + 2 HCl → CuCl2 + H2 2) Ag + FeCl3 → FeCl2 + AgCl
Q: 7.3(a) From information in the Data section, calculate the standard Gibbs energy and the equilibrium…
A:
Q: Calculate the standard Gibbs energy of reaction for 4 HI(g) + O2(g) → 212(s) + 2H2O(1) at 298 K,…
A:
Q: The standard Gibbs energy of formation of PH3 (g) is 13.4 kJ/mol at 298 K. What is the corresponding…
A: Given data,∆Go=13.4kJ/molPH2=1.0barPPH3=0.60bar
Q: The standard Gibbs energy of formation of PH3(g) is +13.4 kJ mol−1 at 298 K. What is the…
A: The reaction for the formation of PH3:Ps+32H2g→PH3gWe use the following formula to calculate gibbs…
Q: Use standard Gibbs energies of formation to calculate the standard reaction Gibbs energies at 298 K…
A: Given : Chemical equation : Zn(s)+Cu2+(aq)→Zn2+(aq)+Cu(s) C12H22O11(s)+12O2(g)→12CO2(g)+11H2O(l)…
Q: Using the information in the data section, calculate the standard reaction ent and standard reaction…
A: Given reaction is : N2 (g) + 3H2 (g) ---------> 2NH3 (g) a). Calculate the standard change in…
Q: Express the relation among the conductivity of solution in the cell, the cell constant and the…
A: Conductivity: It is the measure of the ability of a solution to conduct electricity. Cell constant:…
Q: how to calculate the equilibrium constant given Delta Grxn= +6.4kJ/mol at 298K
A: The relationship between the ΔGrxn and equilibrium constant is given by RTln(K) = -ΔGrxn where R =…
Q: By how much does the cell potential change when Q is increased by a factor of 5 for a reaction in…
A: Formula Ecell = Ecello -0.05913logQ E2-E1 = -0.05913 ( logQ2 -logQ1) = -0.05913 (…
Q: Certain bacteria in the soil obtain the necessary energy for growth by oxidizing nitrite to r…
A: Gibbs energy released can be calculated using the formula given. The standard free energy of Oxygen…
Step by step
Solved in 4 steps with 3 images

- Certain bacteria in the soil obtain the necessary energy for growth by oxidizing nitrite to r nitrate: 2NO2- (aq) + O2(g) —> 2NO3-(aq) Given that the standard Gibbs energies of formation of NO2- and NO3- are -34.6 kJ mol-1 and -110.5 kJ mol-1, respectively, calculate the amount of Gibbs energy released when 1 mole of No2- is oxidized to 1 mole of NO3-.The standard Gibbs energy of formation of NH3(g) is −16.5 kJ mol−1 at 298 K. What is the corresponding reaction Gibbs energy when the partial pressures of the N2, H2, and NH3 (treated as perfect gases) are 3.0 bar, 1.0 bar, and 4.0 bar, respectively? What is the spontaneous direction of the reaction in this case?For PbI2(s) ⇋ Pb2+(aq) + 2 I−(aq), K = 1.4 × 10−8 at 25 °C and the standard Gibbs energy of formation of PbI2(s) is −173.64 kJ mol−1. Calculate the standard Gibbs energy of formation of PbI2(aq).
- The standard Gibbs energy of the reaction N2(g) + 3 H2(g) → 2 NH3(g) is −32.9 kJ mol−1 at 298 K. What is the value of ΔrG when Q = (i) 0.010, (ii) 1.0, (iii) 10.0, (iv) 100 000, (v) 1 000 000? Estimate (by interpolation) the value of K from the values you calculate. What is the actual value of K?The standard Gibbs energy of formation of PH3(g) is +13.4 kJ mol−1 at 298 K. What is the corresponding reaction Gibbs energy when the partial pressures of the H2 and PH3 (treated as perfect gases) are 1.0 bar and 0.60 bar, respectively? What is the spontaneous direction of the reaction in this case?The standard Gibbs energy of formation of PH3 (g) is 13.4 kJ/mol at 298 K. What is the corresponding reaction Gibbs energy when the partial pressures of the H2 and PH3(treated as perfect gases) are 1.0 bar and 0.60 bar, respectively? What is the spontaneous direction of the reaction in this case?
- The standard potential of the AgCl/Ag,Cl− couple fits the expressionE⦵/V = 0.23659 − 4.8564 × 10−4(θ/°C) − 3.4205 × 10−6(θ/°C)2 + 5.869 × 10−9(θ/°C)3Calculate the standard Gibbs energy and enthalpy of formation of Cl−(aq) and its standard entropy at 298 K.Chemistry Using the information in the data section, calculate the standard reaction ent and standard reaction Gibbs energy for the n2(g)+3h2(g) → 2nh3(g) reaction.Carbon may exist as diamond, graphite, and other allotropes. The standard Gibbs energy of formation of diamond is 2.900 kJ mol-1, greater than that of graphite at 298 K. Predict which of these two allotropes is the more stable at this temperature.
- The decomposition of a generic diatomic element in its standard state is represented by the equation 1/2 X2(g)⟶X(g) Assume that the standard molar Gibbs energy of formation of X(g) is 4.34 kJ·mol−1 at 2000. K and−50.82 kJ·mol−1 at 30003000. K. Determine the value of the thermodynamic equilibrium constant, K, at each temperature. At 2000. K, ΔGf=4.34 kJ·mol−1. What is K at that temperature?One enzyme-catalysed reaction in a biochemical cycle has an equilibrium constant that is 8.4 times the equilibrium constant of a second reaction. If the standard Gibbs energy of the former reaction is -250 kJ mol-1, what is the standard reaction Gibbs energy of the second reaction?The chemical potential of a particular substance in the region A of a system is 5kJ higher than potential of this substance in region B. By how much does the Gibbs energy of the system change when 2mmol of the substance is transferred from region A to region B? Will this transfer be spontaneous?

