Q 1: For a particular gas system, the internal energy is given by : U = 2.5 PV + C, where P: Presure, V: volume and C: constant, the sysytem is taken into cycle shown in fig.(Q1) below with three Processes, Calculate Q & W if the pressure is given by P= 10 + 10° (v-0.02)² ? 04 03
Q 1: For a particular gas system, the internal energy is given by : U = 2.5 PV + C, where P: Presure, V: volume and C: constant, the sysytem is taken into cycle shown in fig.(Q1) below with three Processes, Calculate Q & W if the pressure is given by P= 10 + 10° (v-0.02)² ? 04 03
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
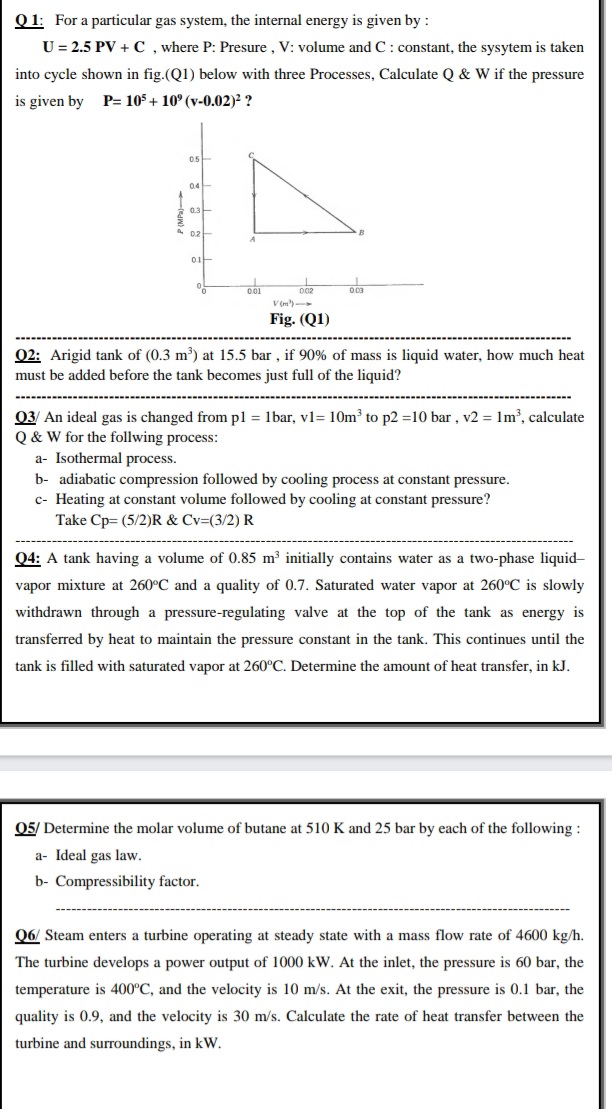
Transcribed Image Text:Q 1: For a particular gas system, the internal energy is given by :
U = 2.5 PV + C , where P: Presure , V: volume and C: constant, the sysytem is taken
into cycle shown in fig.(Q1) below with three Processes, Calculate Q & W if the pressure
is given by
P= 105 + 10° (v-0.02)² ?
05
04
V (m')
Fig. (Q1)
02: Arigid tank of (0.3 m) at 15.5 bar , if 90% of mass is liquid water, how much heat
must be added before the tank becomes just full of the liquid?
03/ An ideal gas is changed from pl = 1bar, vl= 10m³ to p2 =10 bar , v2 = 1m², calculate
Q & W for the follwing process:
a- Isothermal process.
b- adiabatic compression followed by cooling process at constant pressure.
c- Heating at constant volume followed by cooling at constant pressure?
Take Cp= (5/2)R & Cv=(3/2) R
Q4: A tank having a volume of 0.85 m³ initially contains water as a two-phase liquid-
vapor mixture at 260°C and a quality of 0.7. Saturated water vapor at 260°C is slowly
withdrawn through a pressure-regulating valve at the top of the tank as energy is
transferred by heat to maintain the pressure constant in the tank. This continues until the
tank is filled with saturated vapor at 260°C. Determine the amount of heat transfer, in kJ.
05/ Determine the molar volume of butane at 510 K and 25 bar by each of the following :
a- Ideal gas law.
b- Compressibility factor.
06/ Steam enters a turbine operating at steady state with a mass flow rate of 4600 kg/h.
The turbine develops a power output of 1000 kW. At the inlet, the pressure is 60 bar, the
temperature is 400°C, and the velocity is 10 m/s. At the exit, the pressure is 0.1 bar, the
quality is 0.9, and the velocity is 30 m/s. Calculate the rate of heat transfer between the
turbine and surroundings, in kW.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY