Q: What effect will adding more SO3 have on the system
A:
Q: onstruct the expression for Kc for the llowing reaction. 2 Ag(s) + Zn*(aq) =2 Ag*(aq) + Zn(s) 1 Drag…
A:
Q: Consider the hypothetical cations, A' and C* and anions, B and D'. Calculate the AG when 20.0 mL of…
A:
Q: 7) For a reaction Keg = 1.2 x 10-6 at T = 200 K. What is AG° for the reaction?
A:
Q: What is the quilibrium constant for a reaction at 25oC. The value of Delta G is -56.8 kJ/mol?
A: To Calculate the Keq follow the equation
Q: A student prepares a stock solution of BaF2 by dissolving 50.00g of a BaF2 in 1.00L of water. An…
A: Given data,Mass of BaF2=50.00gVolume of water=1.00LMolarity of desired solution=0.100MVolume of…
Q: As a result of breakdown of the Ventilation system, the gaseous product of the reaction of 2 kg Of…
A: A balanced reaction is one that has equal number of atoms of each element on both reactants and…
Q: For the reaction Sm(s) + 2Cu? (aq) -› Sn?*(ag) + 2Cu* (aq) the calculated E° = +0.291V, calculate…
A:
Q: Calculate E° that would be developed by the cells using the following reactions (all concentrations…
A: The cell potential is calculated by the reduction potential of the electrode. If we know the…
Q: A precipitate of AgCl + AgBr weighs 0.8132 g. On heating on a current of Cl2, the AgBr is converted…
A: The mass percentage is one of the most important method to determine the concentration of a…
Q: In nonaqueous solvents, it is possible to react HF to create H2F+. Which of these statements follows…
A: The given observation is that in nonaqueous solvents, it is possible to react HF to create H2F+ . It…
Q: Calculate Kp for each salt in an aqueous solution at 25 °C in which u = 0.0100 M. Use the table of K…
A: The solubility product constant (Ksp) represents the solubility of products at equilibrium for…
Q: Tetraethyl lead, Pb(C.H5)4, in a 25.00 mL sample of jet fuel was shaken with 15.00 mL of 0.02095 M…
A: To calculate percent of Pb(C2H5)4 in sample , we need to calculate it's mass . For this we would…
Q: :The electrolyte solution within the glass electrode (ref) of the pH meter is .a O Dilute KC1…
A: Glass electrode made up of glass tube with a thin glass bulb at the tip.
Q: Calculate the (a) electrical energy, (b) AG°, and (c) K of the reaction:
A: Standard electrode potential is the measure of electrode potential measured under standard…
Q: At 20 °C a saturated solution of silver acetate, AgCh3CO2 contains 2.4 g of the silver compound…
A: At 20 °C, 2.4 g of silver compound is dissolved in 240.0 mL of silver acetate, AgCH3CO2 solution…
Q: Waste acid of pH 3.0 is stored in a lead-lined vessel, as shown in the figure below. If the walls of…
A: The pH is a measure of the power of proton in a given concentration. The equation for pH is ,…
Q: Calculate the molar concentration of zinc ions in a solution containing 0.200 M [Zn(NH3)4]2+ (Kf =…
A:
Q: What is the value of K for this aqueous reaction at 298 K? A +B=C+ D AG = 17.70 kJ/mol %3D
A: The relationship between standard Gibbs energy change (∆G°) , temperature (T) and equilibrium…
Q: Approximate the relative amounts of A and B in the figure. Calculate the equilibrium constant K.…
A: According to the graph, at the beginning, there was only A. As the reaction proceeds, A converts to…
Q: calculate the following: Vos = 20.20 ml (Cro.') = 0.1350 M (Ag') = 0.202 M Kıp = 1.17x 101 M a)…
A: (a) Answer - According to the question - Given - Balanced equation - CrO42- + 2Ag+ ------->…
Q: A series of reactions is carried out on the alloy (A) formed of two metals, as in the following…
A:
Q: In the following reaction calculate and find the normality when it is 1.0 M H3PO4? H3PO4 + 2NaOH →…
A: Given: Molarity of H3PO4 = 1.0 M
Q: Which of the following statements is incorrect? O In metallic indicator electrodes, the potential…
A: A multiple choice question based on electrochemistry that is to be accomplished.
Q: What is the value of K for this aqueous reaction at 298 K Delta G=28.66 A+B<---->C+D
A: One kilojoule is equal to 1000 joules. So, (1000J/1kJ) is used as a conversion factor.Rearrange the…
Q: Calculate K for each salt in an aqueous solution at 25 °C in Compound Formula which u = 0.0100 M.…
A:
Q: Calculate the (a) electrical energy, (b) AG°, and (c)K of the reaction:
A: Electrical energy is defined as the energy possessed by charged particles. In the electrochemical…
Q: Zinc(s) (MW=65.38), with a mass of 0.825 g, was reacted with 25.00 mL of NaClO (MW=74.44) according…
A: We first balance the given redox reaction in a basic medium using the half-reaction method. We…
Q: If Kc = 0.0092 for the reaction of Kp? %D 3 A (a) + B (g) =C (g) + D (g)
A: Unsjs
Q: 2- A cell in a Consisting of 0.lm and 00lm silver plates dipping solution of silver nitrate apping…
A: Let us discuss the question. This is a type of concentration cell.
Q: What is the relationship between Kp and Kc for the reaction, 2ICl(g) I2(g) + Cl2(g)?
A:
Q: Write balanced equations for the following redox reactions: a. NaBr + Cl2 -> NaCl + Br2 b. Fe2O3 +…
A: We have to balance equations for the following redox reactionsa. NaBr + Cl2 -> NaCl + Br2 b.…
Q: At 25 o C: 3Pe2 (s) + 2Fe (s) -> 2Fe3+ (aq) + 6Pe- (aq) Eo(Pe2/Pe-) = +0.55 V Eo(Fe3+/Fe) =…
A: Given, Temperature (T) = 25 o C: 3Pe2 (s) + 2Fe (s) -> 2Fe3+ (aq) + 6Pe- (aq) Standard reduction…
Q: 1. Balance the following reactions in both acidic and basic media, identify the oxidation and…
A: A chemical reaction in which oxidation and reduction take place simultaneously is called a redox…
Q: 2. At 25°C, K= 399 for the following reaction: Br + AgCls) → Cľ + AgBr(s) [CI" is independent from…
A: The reaction given is, => Br- (aq) + AgCl (s) --------> Cl- (aq) + AgBr (s) Given: At 25 oC,…
Q: The solubility-product constant for Ag2SO3 is 1.5x10-14. Calculate E0 for the process
A:
Q: 7. Calculate AG° for the reaction 3NO2 (g) + H20 (I) = 2HNO3 (I) + NO (g). AG, (kJ/mol) -237.2…
A: To find the ∆G° for the given reaction , we can use the formula ;
Q: What is the value of the equilibrium constant for the reaction. 2 Agis) + Pt2 (aq) Ag (aq) + Pt(s)…
A:
Q: 5. Tetraethyl lead, Pb(C:Hs)4, in a 25.00 mL sample of jet fuel was shaken with 15.00 mL of 0.02095…
A: In an acid-base titration the equivalence point is defined as the mixing of chemically equivalent…
Q: t is to be reduced to nickel metal in an industrial process by the reaction: NiO(s) -------->…
A: Since the equation is giving as wrong . But i do it with the right equation. Redox is a kind of…
Q: Calculate the ionic strength (u) for each of the aqueous solutions. Assume 100% dissociation of the…
A: According to the question, we need to determine the ionic strength of certain compounds. The…
Q: The overall reaction for the corrosion of iron (rusting) by oxygen is…
A:
Q: A copper storage tank containing dilute H,SO, at pH 0.1 is blanketed with hydrogen at 1 atm.…
A: The standard potential for the reduction of Cu is obtained from the table as 0.342V Cu+2 + 2e →Cu…
Q: Consider two solutions, the first being 50.0 mL of1.00 M CuSO4 and the second 50.0 mL of 2.00 M…
A: “Since you have posted a question with multiple sub-parts, we will solve first three sub-parts for…
Q: The solubility product constant for Copper(II)phosphate, Cu (PO ) , is 1.3 x 10-37 at 25°C. 342(a)…
A: Any substance’s maximum quantity that can be mixed into a known solvent to yield the saturated…
Q: Balance the following reaction in basic solution. Ag(s) + Zn?+ (ag) → Ag,0(ag) + Zn(s) Fill in the…
A: The chemical equations should be balanced in accordance with the law of conservation of mass. This…
Q: What is a nice laboratory introduction about the determination of E.M.F. of a cell?
A:
Q: om the following data calculate the normalities of the acid and base solutions: weight of pure…
A: Here, Nax 0.982= Nbx 1ml Acid as, 0.2448105.98 =Va ×Na2.31×10-3 =Va ×Na Then, 0.84Nb =43.65-VaNa
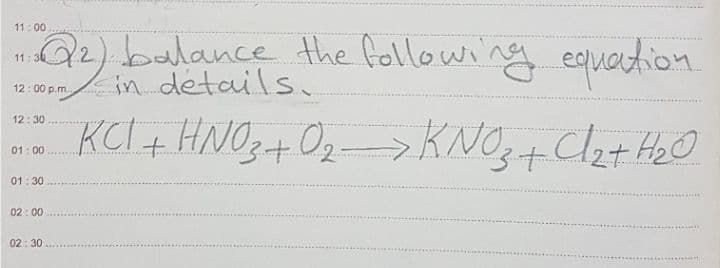
Step by step
Solved in 2 steps

- Consider an urban atmosphere containing 100 ppbv NOx and 100 ppbv O3 with T=298K and P=1 atm. NO + O3 --> NO2 + O2 (1) NO2 + hv --> NO + O3 (in the presence of O2) (2) With k1=2.2x10-12 exp(-1430/T) cm3 molecule-1s-1 and k2=1x10-2 s-1 (ie, at noon). Calculate the steady-state concentration of NO and NO2 at noon based on the above reactions.hello, i sent this question before but the answer you rpovided for both wasnt right and i couldnt undertstand how you got the answer. can you please show me a handwritten solution for better understanding. 1a) The decomposition of ethanol at some constant temperature (above 500°C), over a copper surface, C2H5OH(g) CH3CHO(g) + H2(g) was studied by monitoring the total pressure with time.The following data were obtained: t (s) Ptotal (torr) 0 120 45 131 139 154 233 177 376 212 380 213 What will be the total pressure at t = 462 s? 1b) What is the rate constant (k)?(Include appropriate units.)A3 Given that Given that the Butler-Volmer equation imeas = io {exp (αnFηa)/RT) - exp [- (1-α)nFηa)/RT)]}. Show the derivation of Tafel equation.
- The degradation of CF3CH2F (an HFC) by OH radicals inthe troposphere is first order in each reactant and has arate constant of k = 1.6 x 108 M-1s-1 at 4 °C. If the troposphericconcentrations of OH and CF3CH2F are 8.1 x 105and 6.3 x 108 molecules/cm3, respectively, what is the rateof reaction at this temperature in M/s?The rate of photodecomposition of the herbicide piclo- ram in aqueous systems was determined by exposure to sunlight for a number of days. One such experiment produced the following results. (Data from R.T. Hedlun and C.R. Youngson, “The Rates of Photodecomposition of Picloram in Aqueous Systems," Fate of Organic Pesticides in tbe Aquatic Environment, Advances in Chemistry Series, #111, American Chemical Society (1972), 159—172.) Exposure Time, t (days) [Pidoram] (mol L_1) 0 4.14 X 10-6 7 3.70 X 10-6 14 3.31 X 10-6 21 2.94 X 10~6 28 2.61 X 10~6 35 2.30 X 10-6 42 2.05 X 10-6 49 1.82 X 10"6 56 1.65 X 10-6 Determine the order of reaction, the rate constant, and the half-life for the photodecomposition of picloram.The peroxydisulfate ion (S2O8-2) reacts with the iodide ion in aqueous solution via the reaction: S2O82-(aq) + 3I- → 2SO4(aq)+ I3-(aq). An aqueous solution containing 0.050 M of S2O8-2 ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. Time (s) 0.00 400.0 800.0 1200.0 1600.0 [I‑] (M) 0.072 0.057 0.046 0.037 0.029 Determine the concentration of S2O82- remaining at 400 s in M. Determine the concentration of S2O82- remaining at 800 s in M. Determine the concentration of S2O82- remaining at 1600 s in M.
- The peroxydisulfate ion (S2O8-2) reacts with the iodide ion in aqueous solution via the reaction: S2O82-(aq) + 3I- → 2SO4(aq)+ I3-(aq). An aqueous solution containing 0.050 M of S2O8-2 ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. Time (s) 0.00 400.0 800.0 1200.0 1600.0 [I‑] (M) 0.072 0.057 0.046 0.037 0.029 Determine the average rate of disappearance of I- between 400.0 s and 800.0 s in M/s. Determine the average rate of disappearance of I- in the initial 400.0 s in M/s. Determine the average rate of disappearance of I- between 1200.0 s and 1600.0 s in M/s. Determine the concentration of S2O82- remaining at 400 s in M. Determine the concentration of S2O82- remaining at 800 s in M. Determine the concentration of S2O82- remaining at 1600 s in M.Provided the reaction A + 2B + C → 2D + E, and the set of data thatfollows:Consider the reaction Mn+ + ne- ⇌ M. If ΔG*c = ΔG* + (1-α)nFE and η = E – Eeq, prove that ic = io exp[-(1-α)nFηa)/RT)].
- Specify the pre-equilibrium and steady-state approximations and explain why they might lead to different conclusions.given that the cricket chirps 179 times per minute at 25.0oC and 142 times per minute at 21.7oC, determine Ea for the reaction controlling the chirping.The atomic hydrogen exists in space at an estimated concentration of one particle per cubic meter. If the collision diameter is 2.5 ×10^(–10) meter and the temperature is 2.7 Kelvin, how many kilometers away will the next potential collision be?



