Q2 For the chemical reaction shown below select the correct the equilibrium constant expression for Kp. 4NH3(9) + 302(g) = 2N2/9) + 6H2O(g) Q3 4NH3(0) * 3020) = 2N2(9) * 6H2O(@) If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen (02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2) gas then how many moles of ammonia (NH3) is present at equilibrium? 4NH3(0) + 302(9) 2N2(9) + 6H20(@) Q4 If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen (02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2) gas then how many moles of water (H,O) is present at equilibrium?
Q2 For the chemical reaction shown below select the correct the equilibrium constant expression for Kp. 4NH3(9) + 302(g) = 2N2/9) + 6H2O(g) Q3 4NH3(0) * 3020) = 2N2(9) * 6H2O(@) If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen (02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2) gas then how many moles of ammonia (NH3) is present at equilibrium? 4NH3(0) + 302(9) 2N2(9) + 6H20(@) Q4 If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen (02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2) gas then how many moles of water (H,O) is present at equilibrium?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 51QRT: At room temperature, the equilibrium constant Kc for the reaction
2 NO(g) ⇌ N2(g) + O2(g)
is 1.4 ×...
Related questions
Question
All these questions are related, It's one question. If you are not going to answer them all, cancel the question.

Transcribed Image Text:Q2
For the chemical reaction shown below select the correct the equilibrium constant expression for
4NH3(g) + 302(g) = 2N2(9) + 6H2O(g)
Q3
4NH3(9) + 302(9) = 2N2(9) + 6H2O(9)
If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen
(02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes
at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2)
gas then how many moles of ammonia (NH3) is present at equilibrium?
4NH3(9) + 302(9) 2N2(9) + 6H2O(g)
Q4
If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen
(02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes
at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2)
gas then how many moles of water (H,O) is present at equilibrium?
4NH3(9) + 302(g) 2N2(9) + 6H2O(9)
Q5
If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen
(02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes
at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2)
gas then what is the Molarity (M) of oxygen (02) gas present at equilibrium?
4NH3(9) + 302(g) = 2N2(9) + 6H20(g)
Q6
If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen
(02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes
at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2)
gas.
Now that you have the moles and Molarity of each reactant and product calculate the
Kc value for this chemical equilibrium reaction.
4NH3(9) + 302(9) = 2N2(9) + 6H2O(9)
Q7
If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen
(02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes
at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2)
gas.
Now that you have calculated the Kc value use the equation K, = Ke (RT)A" to solve
for the Kp value.
Transcribed Image Text:4NH39) + 302(g) = 2N2(9) + 6H2O(9)
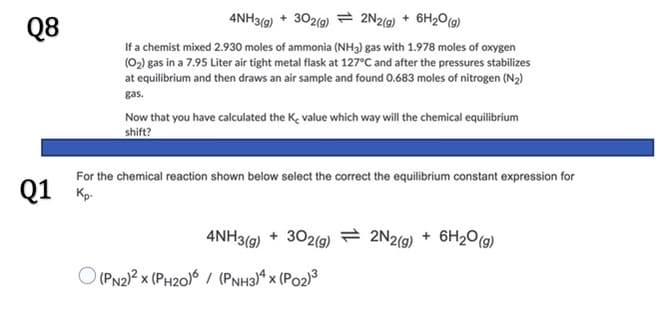
Q8
If a chemist mixed 2.930 moles of ammonia (NH3) gas with 1.978 moles of oxygen
(02) gas in a 7.95 Liter air tight metal flask at 127°C and after the pressures stabilizes
at equilibrium and then draws an air sample and found 0.683 moles of nitrogen (N2)
gas.
Now that you have calculated the Ke value which way will the chemical equilibrium
shift?
For the chemical reaction shown below select the correct the equilibrium constant expression for
Q1 K
4NH3(9) + 302(9)
2 2N2(9)
6H20(g)
+
O (PN2)? x (PH20)6 / (PNH3)ª x (Po2)3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning