Q2. (a) Give the names of parts A and B in the viscometer Mark X Bulb B Mark Y Bulb A A B Q2. The following data were obtained for an experiment to determine the density of a turpentine oil: -Mass of empty pycnometer = 20.74g Mass of pycnometer and distilled water 55.59g -The density of water at room temperature = 0.997g/mL - Mass of empty pycnometer and turpentine oil= 50.39 g Mz M z Z0.79 g Determine the density of a turpentine oil:
Q2. (a) Give the names of parts A and B in the viscometer Mark X Bulb B Mark Y Bulb A A B Q2. The following data were obtained for an experiment to determine the density of a turpentine oil: -Mass of empty pycnometer = 20.74g Mass of pycnometer and distilled water 55.59g -The density of water at room temperature = 0.997g/mL - Mass of empty pycnometer and turpentine oil= 50.39 g Mz M z Z0.79 g Determine the density of a turpentine oil:
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 100AE: The active ingredient of aspirin tablets is acetylsalicylic acid, which, has a density of 1.4 g/cm3....
Related questions
Question
Please solve this
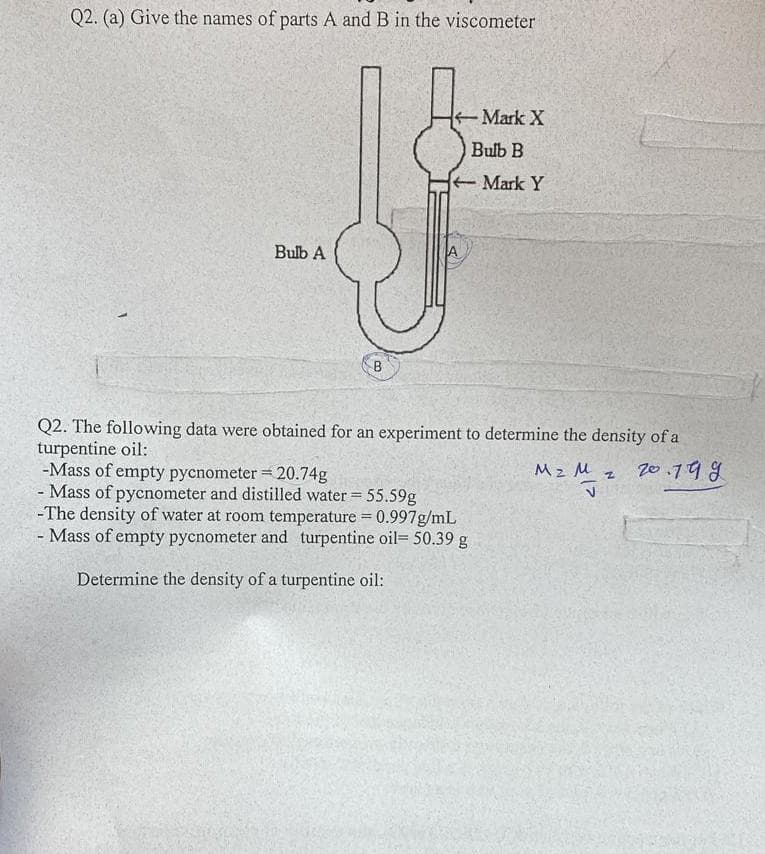
Transcribed Image Text:Q2. (a) Give the names of parts A and B in the viscometer
Mark X
O Bulb B
Mark Y
Bulb A
B
Q2. The following data were obtained for an experiment to determine the density of a
turpentine oil:
-Mass of empty pycnometer = 20.74g
- Mass of pycnometer and distilled water 55.59g
-The density of water at room temperature = 0.997g/mL
- Mass of empty pycnometer and turpentine oil= 50.39 g
Mz M z 2o.798
Determine the density of a turpentine oil:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning