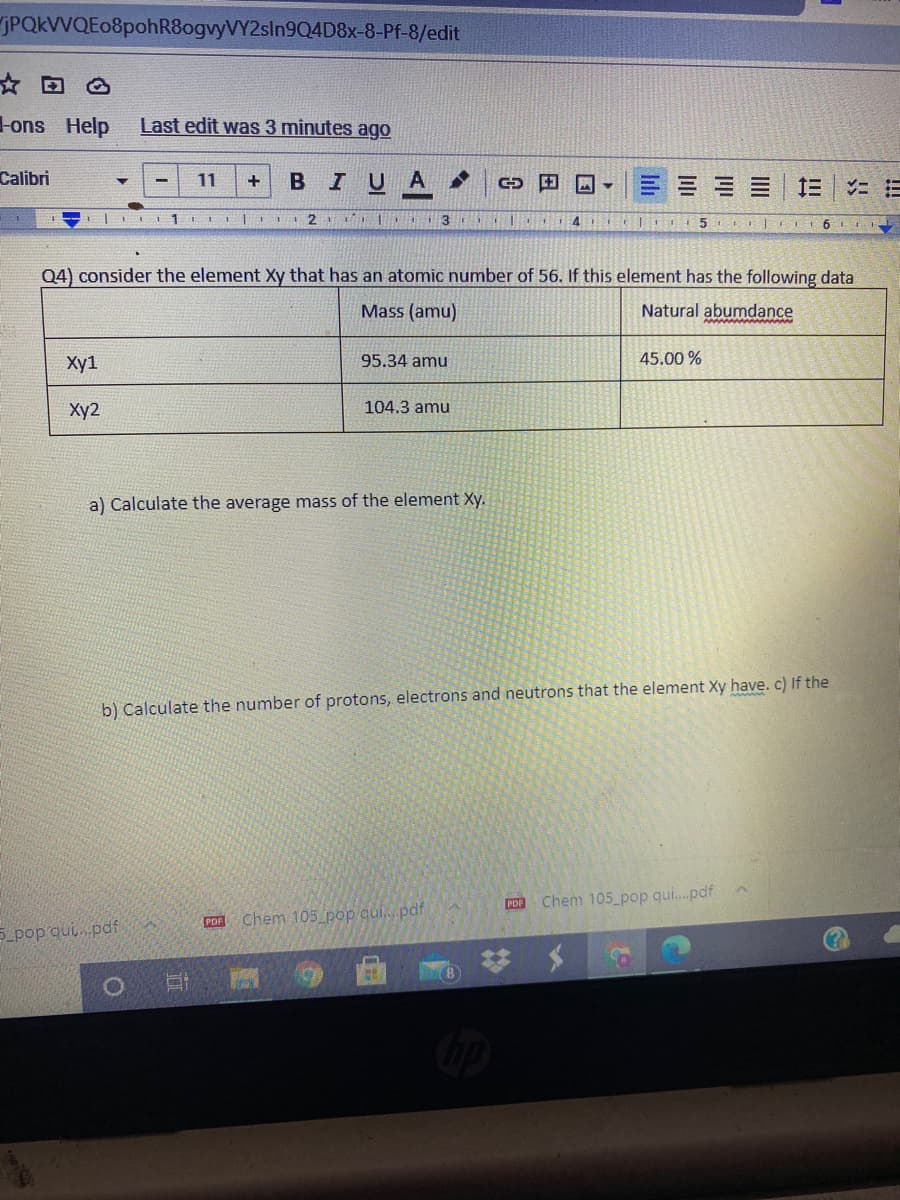
Q4) consider the element Xy that has an atomic number of 56. If this element has the following data Mass (amu) Natural abumdance Xy1 95.34 amu 45.00 % Ху2 104.3 amu
Q4) consider the element Xy that has an atomic number of 56. If this element has the following data Mass (amu) Natural abumdance Xy1 95.34 amu 45.00 % Ху2 104.3 amu
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
Section: Chapter Questions
Problem 11STP
Related questions
Question
Transcribed Image Text:jPQkVVQEo8pohR8ogvyVY2sln9Q4D8x-8-Pf-8/edit
-ons Help
Last edit was 3 minutes ago
Calibri
11
BIUA
D 田国▼
=|三
3
4 5 | 6I
Q4) consider the element Xy that has an atomic number of 56. If this element has the following data
Mass (amu)
Natural abumdance
Хy1
95.34 amu
45.00 %
Xy2
104.3 amu
a) Calculate the average mass of the element Xy.
b) Calculate the number of protons, electrons and neutrons that the element Xy have.c) If the
PDF
Chem 105_pop qui...pdf
PDF
Chem 105 pop qui. pdf
5_pop qui. pdf
8.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning