Question 10 Solid CO2 (0.33 mol) is reacted with CH3MBB (0.23 mol) in ether solvent. The product of the reaction is isolated in aqueous solution (assume 100% yield) and excess CO2 is removed. Subsequently, HCI (aq) (0.10 mol) is added. What is the pH of the solution that results? Notes: You will need to look up a pK, for this question and report your answer to 2 decimal places. A linear dialkene has 7 carbon atoms. If excess HI (> 2.0 mol equivalents) is added a mixture of the alkene, what is the MW of the final product? Answer in g mol to one decimal place.
Question 10 Solid CO2 (0.33 mol) is reacted with CH3MBB (0.23 mol) in ether solvent. The product of the reaction is isolated in aqueous solution (assume 100% yield) and excess CO2 is removed. Subsequently, HCI (aq) (0.10 mol) is added. What is the pH of the solution that results? Notes: You will need to look up a pK, for this question and report your answer to 2 decimal places. A linear dialkene has 7 carbon atoms. If excess HI (> 2.0 mol equivalents) is added a mixture of the alkene, what is the MW of the final product? Answer in g mol to one decimal place.
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
Solve both please...
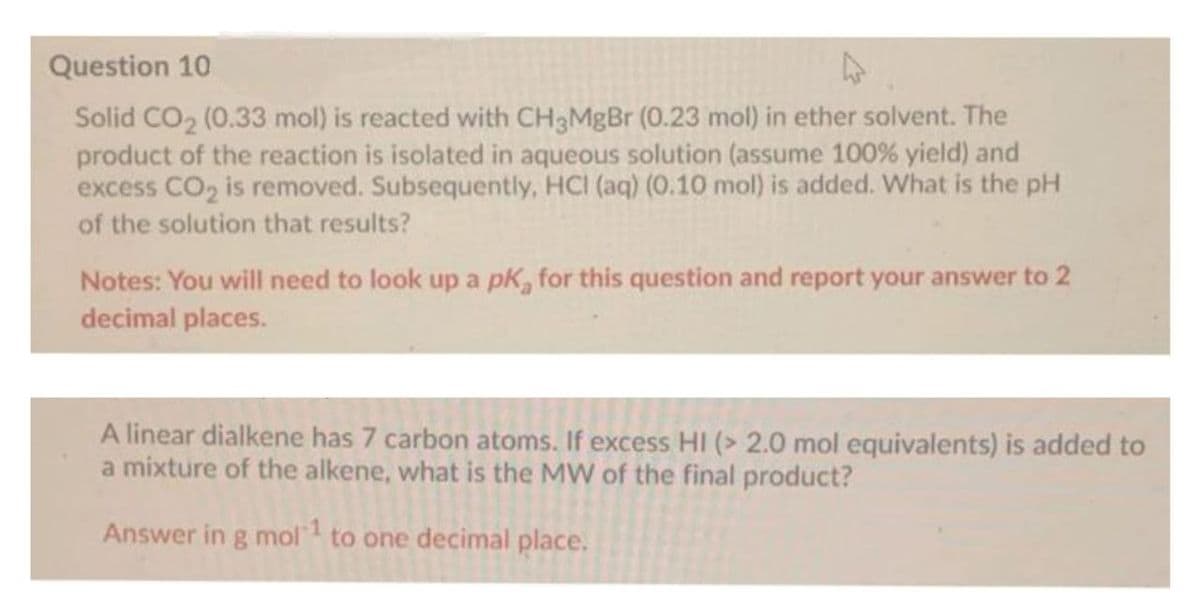
Transcribed Image Text:Question 10
Solid CO2 (0.33 mol) is reacted with CH3MgBr (0.23 mol) in ether solvent. The
product of the reaction is isolated in aqueous solution (assume 100% yield) and
excess CO2 is removed. Subsequently, HCI (aq) (0.10 mol) is added. What is the pH
of the solution that results?
Notes: You will need to look up a pk, for this question and report your answer to 2
decimal places.
linear dialkene has 7 carbon atoms. If excess HI (> 2.0 mol equivalents) is added to
a mixture of the alkene, what is the MW of the final product?
Answer in g mol to one decimal place.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

