• Question 11 The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between sodium bicarbonate, NaHCO, and citric acid, H: CH-0; 3 NaHCO3 (aq) • H;CgH;0; (aq) -> 3 co, (g) - 3 H,0(1) NazCgH507 (aq) H;GgH;0; (aq) -> 3 CO2 (e) In a certain experiment 0.051 moles of sodium bicarbonate and 0.041 moles of citric acid are allowved to react. Which chemical is the limiting reactant? Select an answer Which chemical is the excess reactant? Select an answer How much of the limiting reactant is left over when the reaction is complete? moles How much of the excess reactant is left over when the reaction is complete? moles Submit Question L O U O F5 F6 F7 F10 F11 F12 ScrLk P F3 F4 FB F9 & 3 4 5 6 8 9. R Y U I D H. JK C VB M 目 Alt Ctrl
• Question 11 The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between sodium bicarbonate, NaHCO, and citric acid, H: CH-0; 3 NaHCO3 (aq) • H;CgH;0; (aq) -> 3 co, (g) - 3 H,0(1) NazCgH507 (aq) H;GgH;0; (aq) -> 3 CO2 (e) In a certain experiment 0.051 moles of sodium bicarbonate and 0.041 moles of citric acid are allowved to react. Which chemical is the limiting reactant? Select an answer Which chemical is the excess reactant? Select an answer How much of the limiting reactant is left over when the reaction is complete? moles How much of the excess reactant is left over when the reaction is complete? moles Submit Question L O U O F5 F6 F7 F10 F11 F12 ScrLk P F3 F4 FB F9 & 3 4 5 6 8 9. R Y U I D H. JK C VB M 目 Alt Ctrl
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
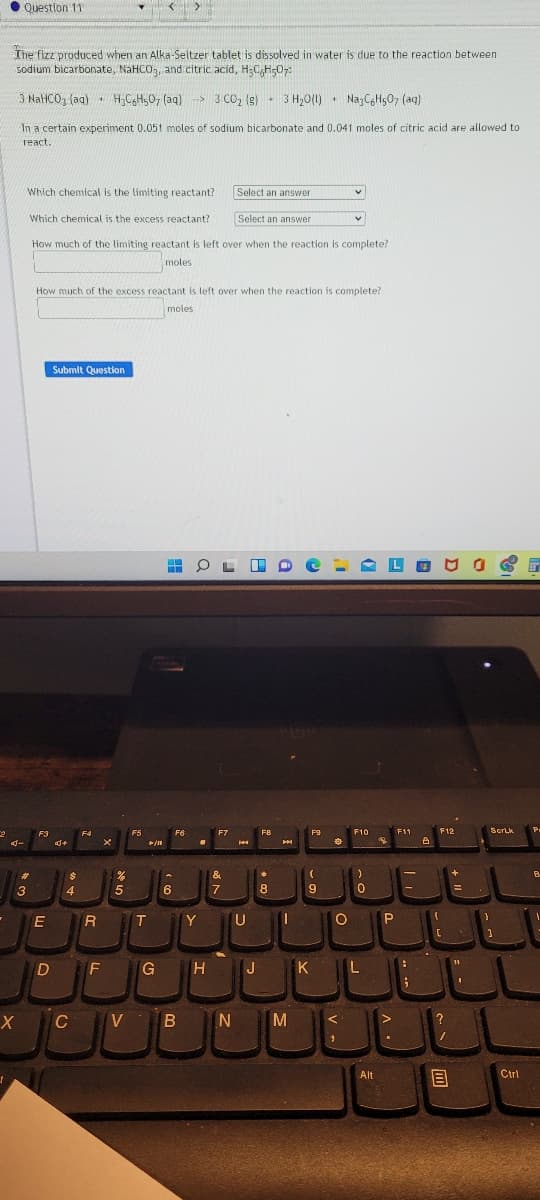
Transcribed Image Text:• Question 11
The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between
sodium bicarbonate, NaHCO, and citric acid, H CH-0;
3 NaHCO, (aa) H,C,H,0, (aq) > 3 CO, (g) - 3 H20(1) • NazCGH507 (aq)
3 H,0(1)
In a certain experiment 0.051 moles of sodium bicarbonate and 0.041 moles of citric acid are allowed to
react.
Which chemical is the limiting reactant?
Select an answer
Which chemical is the excess reactant?
Select an answer
How much of the limiting reactant is left over when the reaction is complete?
moles
How much of the excess reactant is left over when the reaction is complete?
moles
Submit Question
L O U O
LEO
F3
F4
F5
F6
F7
FB
F9
F10
F11
F12
ScrLk
可+
23
&
3
4
5
6
7
8
9.
R
Y
D
H.
JK
C
VB
M
Alt
目
Ctrl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning