Question 12 of 29 > Iodine is prepared both in the laboratory and commercially by adding Cl, (g) to an aqueous solution containing sodium iodide. 2 Nal(aq) +Cl, (g) ,(s)2 NaCl (aq) - How many grams of sodium iodide, Nal, must be used to produce 60.0 g of iodine, I,? mass: g Nal Question Source: MRG- General Chemistry | Publisher: University Science Books help contact us terms of use privacy policy about us careers
Question 12 of 29 > Iodine is prepared both in the laboratory and commercially by adding Cl, (g) to an aqueous solution containing sodium iodide. 2 Nal(aq) +Cl, (g) ,(s)2 NaCl (aq) - How many grams of sodium iodide, Nal, must be used to produce 60.0 g of iodine, I,? mass: g Nal Question Source: MRG- General Chemistry | Publisher: University Science Books help contact us terms of use privacy policy about us careers
Chapter4: Molecules, Compounds, And Chemical Reactions
Section: Chapter Questions
Problem 4SC
Related questions
Question
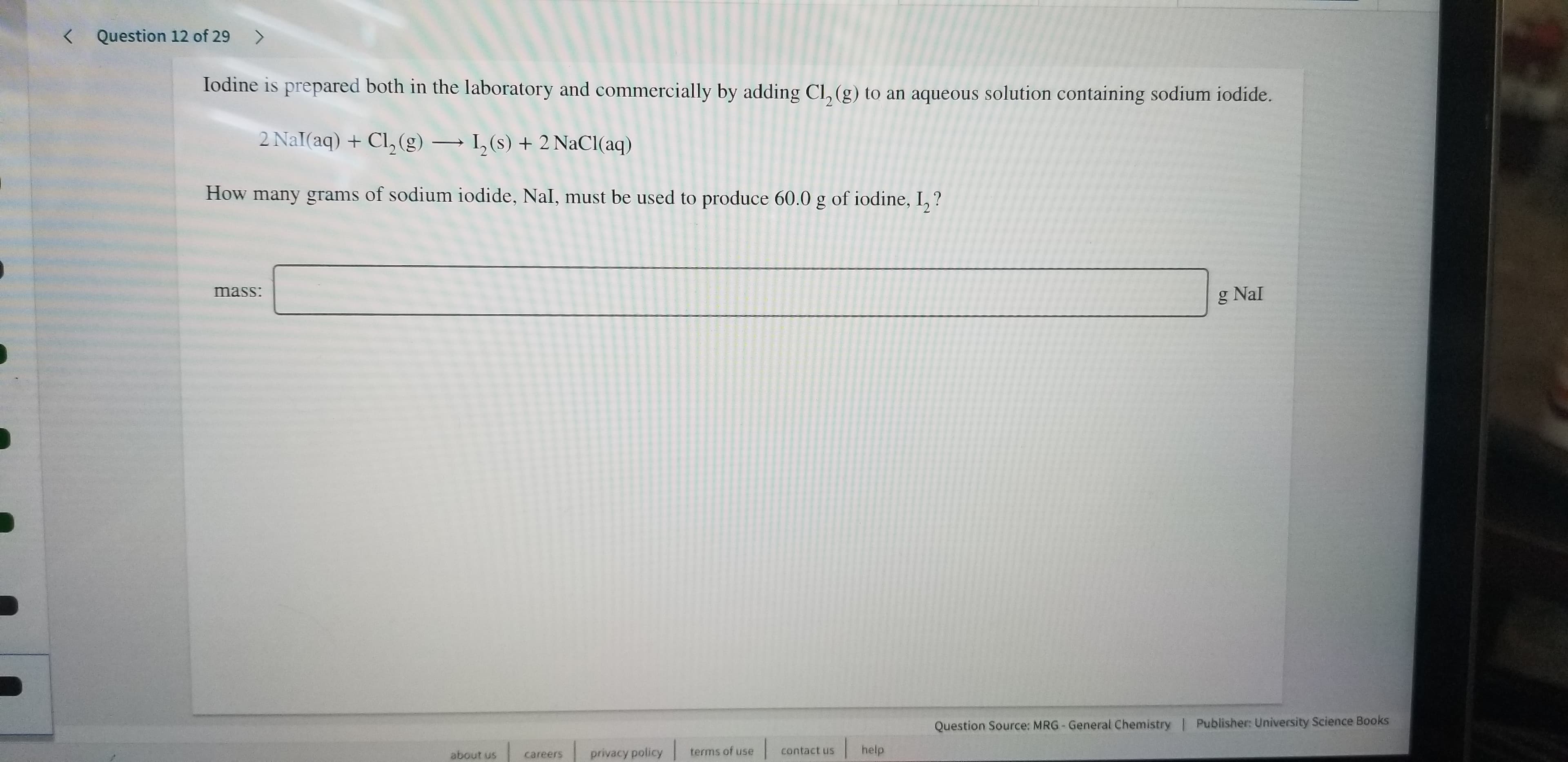
Transcribed Image Text:Question 12 of 29
>
Iodine is prepared both in the laboratory and commercially by adding Cl, (g) to an aqueous solution containing sodium iodide.
2 Nal(aq) +Cl, (g)
,(s)2 NaCl (aq)
-
How many grams of sodium iodide, Nal, must be used to produce 60.0 g of iodine, I,?
mass:
g Nal
Question Source: MRG- General Chemistry | Publisher: University Science Books
help
contact us
terms of use
privacy policy
about us
careers
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 4 images

Recommended textbooks for you



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax