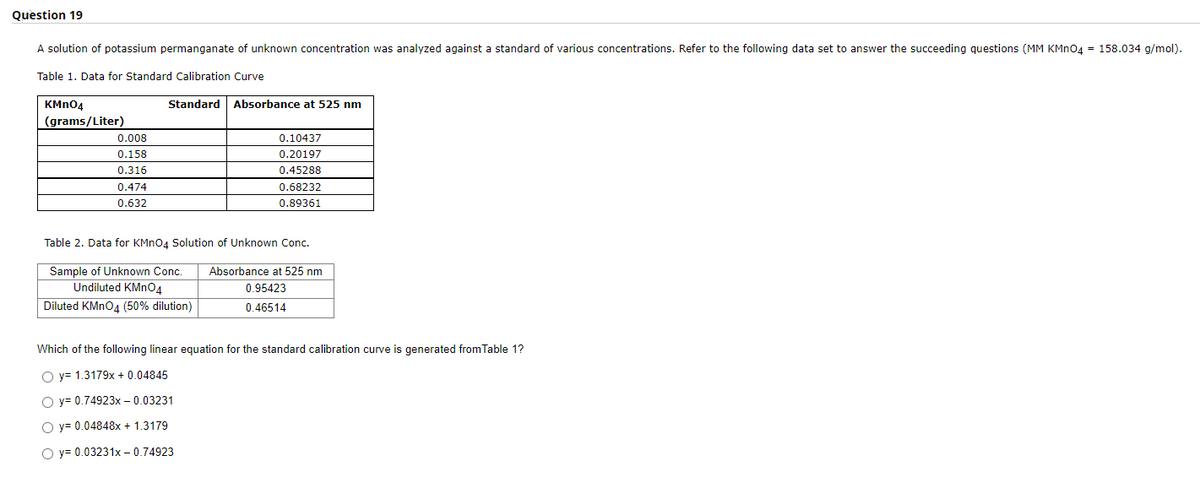
Question 19 A solution of potassium permanganate of unknown concentration was analyzed against a standard of various concentrations. Refer to the following data set to answer the succeeding questions (MM KMNO4 - 158.034 g/mol). Table 1. Data for Standard Calibration Curve KMn04 Standard Absorbance at 525 nm (grams/Liter) 0.008 0.10437 0.158 0.20197 0.45288 0.68232 0.316 0.474 0.632 0.89361 Table 2. Data for KMN04 Solution of Unknown Conc. Sample of Unknown Conc. Undiluted KMNO4 Diluted KMNO, (50% dilution) Absorbance at 525 nm 0.95423 0.46514 Which of the following linear equation for the standard calibration curve is generated from Table 1? O y= 1.3179x + 0.04845 O y= 0.74923x – 0.03231 O y= 0.04848x + 1.3179 O y= 0.03231x - 0.74923
Question 19 A solution of potassium permanganate of unknown concentration was analyzed against a standard of various concentrations. Refer to the following data set to answer the succeeding questions (MM KMNO4 - 158.034 g/mol). Table 1. Data for Standard Calibration Curve KMn04 Standard Absorbance at 525 nm (grams/Liter) 0.008 0.10437 0.158 0.20197 0.45288 0.68232 0.316 0.474 0.632 0.89361 Table 2. Data for KMN04 Solution of Unknown Conc. Sample of Unknown Conc. Undiluted KMNO4 Diluted KMNO, (50% dilution) Absorbance at 525 nm 0.95423 0.46514 Which of the following linear equation for the standard calibration curve is generated from Table 1? O y= 1.3179x + 0.04845 O y= 0.74923x – 0.03231 O y= 0.04848x + 1.3179 O y= 0.03231x - 0.74923
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
Transcribed Image Text:Question 19
A solution of potassium permanganate of unknown concentration was analyzed against a standard of various concentrations. Refer to the following data set to answer the succeeding questions (MM KMNO4 = 158.034 g/mol).
Table 1. Data for Standard Calibration Curve
KMN04
Standard
Absorbance at 525 nm
(grams/Liter)
0.008
0.10437
0.158
0.20197
0.316
0.45288
0.474
0.68232
0.632
0.89361
Table 2. Data for KMno4 Solution of Unknown Conc.
Sample of Unknown Conc.
Absorbance at 525 nm
Undiluted KMN04
0.95423
Diluted KMNO4 (50% dilution)
0.46514
Which of the following linear equation for the standard calibration curve is generated from Table 1?
O y= 1.3179x + 0.04845
O y= 0.74923x – 0.03231
O y= 0.04848x + 1.3179
O y= 0.03231x – 0.74923
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

