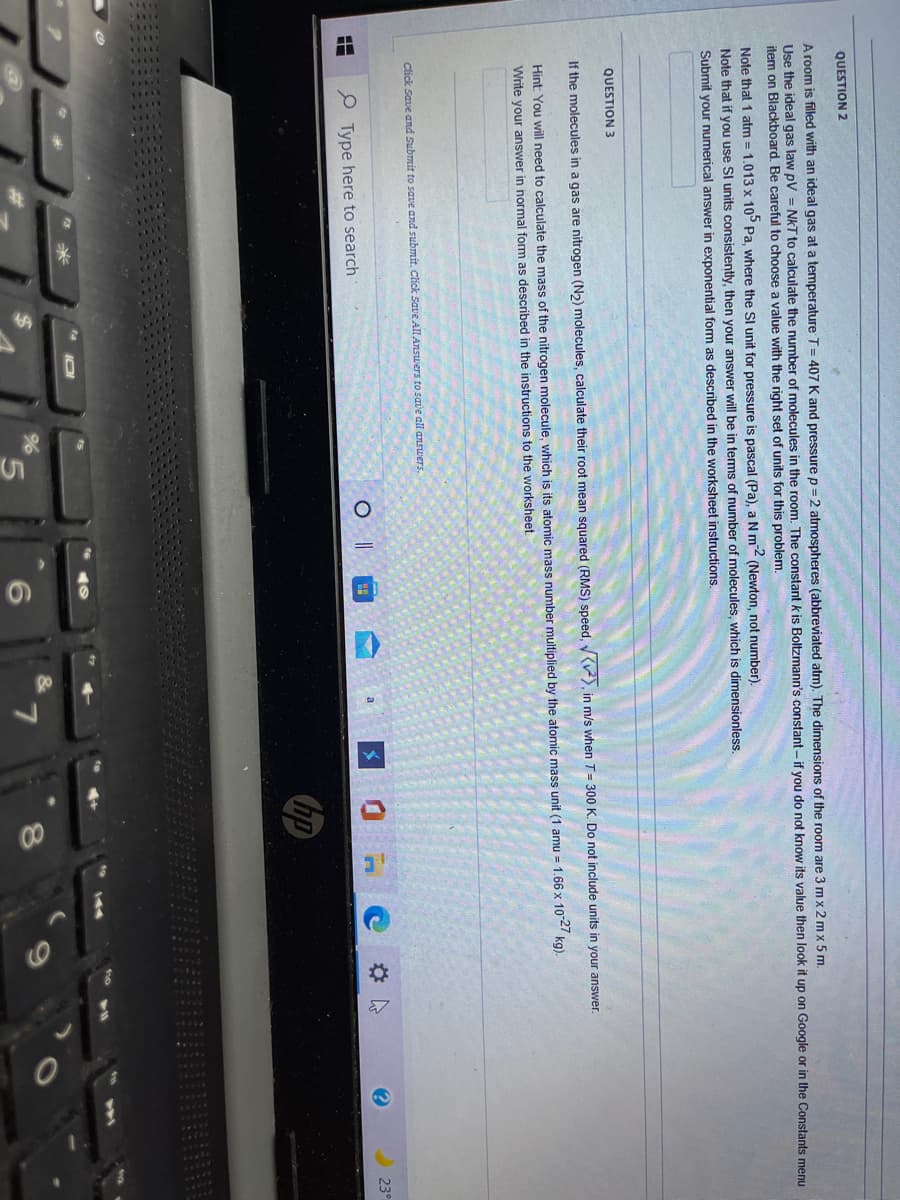
QUESTION 2 A room is filled with an ideal gas at a temperature T= 407 K and pressure p = 2 atmospheres (abbreviated atm). The dime Use the ideal gas law pV = NkT to calculate the number of molecules in the room. The constant k is Boltzmann's constan item on Blackboard. Be careful to choose a value with the right set of units for this problem. %3D %3! Note that 1 atm = 1.013 x 10° Pa, where the SI unit for pressure is pascal (Pa), a N m (Newton, not number). Note that if you use SI units consistently, then your answer will be in terms of number of molecules, which is dimensionles Submit your numerical answer in exponential form as described in the worksheet instructions. %3! QUESTION 3 If the molecules in a gas are nitrogen (N2) molecules, calculate their root mean squared (RMS) speed, (v²), in m/s whi Hint: You will need to calculate the mass of the nitrogen molecule, which is its atomic mass number multiplied by the atom Write your answer in normal form as described in the instructions to the worksheet Click Save and Submit to save and submit. Click Save All Answers to save all answers. P Type here to search
QUESTION 2 A room is filled with an ideal gas at a temperature T= 407 K and pressure p = 2 atmospheres (abbreviated atm). The dime Use the ideal gas law pV = NkT to calculate the number of molecules in the room. The constant k is Boltzmann's constan item on Blackboard. Be careful to choose a value with the right set of units for this problem. %3D %3! Note that 1 atm = 1.013 x 10° Pa, where the SI unit for pressure is pascal (Pa), a N m (Newton, not number). Note that if you use SI units consistently, then your answer will be in terms of number of molecules, which is dimensionles Submit your numerical answer in exponential form as described in the worksheet instructions. %3! QUESTION 3 If the molecules in a gas are nitrogen (N2) molecules, calculate their root mean squared (RMS) speed, (v²), in m/s whi Hint: You will need to calculate the mass of the nitrogen molecule, which is its atomic mass number multiplied by the atom Write your answer in normal form as described in the instructions to the worksheet Click Save and Submit to save and submit. Click Save All Answers to save all answers. P Type here to search
College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter10: Thermal Physics
Section: Chapter Questions
Problem 60AP: A 20.0-L tank of carbon dioxide gas (CO2) is at a pressure of 9.50 105 Pa and temperature of 19.0C...
Related questions
Question
Transcribed Image Text:%2:
QUESTION 2
A room is filled with an ideal gas at a temperature T= 407 K and pressure p = 2 atmospheres (abbreviated atm). The dimensions of the room are 3 mx 2 mx 5 m.
Use the ideal gas law pV = NkT to calculate the number of molecules in the room. The constant k is Boltzmann's constant - if you do not know its value then look it up on Google or in the Constants menu
item on Blackboard. Be careful to choose a value with the right set of units for this problem.
Note that 1 atm = 1.013 x 10° Pa, where the Sl unit for pressure is pascal (Pa), a Nm2 (Newton, not number).
Note that if you use SI units consistently, then your answer will be in terms of number of molecules, which is dimensionless.
Submit your numerical answer in exponential form as described in the worksheet instructions,
QUESTION 3
If the molecules in a gas are nitrogen (N2) molecules, calculate their root mean squared (RMS) speed, y(), in m/s when T= 300 K. Do not include units in your answer.
Hint: You will need to calculate the mass of the nitrogen molecule, which is its atomic mass number multiplied by the atomic mass unit (1 amu = 1.66 x 102 kg).
Write your answer in normal form as described in the instructions to the worksheet
Click Save and Submit to save and submit. Click Save All Answers to save all answerS.
23°
P Type here to search
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning