QUESTION 2 ADP ATP AG"= 1.75 kJ/mol AG"=-18.9 kJ/mol 1,3-Bisphosphoglycerate → 3-Phosphoglycerate Normal free energy of the shown step of glycolysis coupled with synthesis of one molecule of ATP is -18.9 kJ/mol. Calculate AG" (in kJ/mol) of dephosphorylation of 1,3-Biphosphoglycerate if AG" of synthesis of ATP from ADP is 1.75.
QUESTION 2 ADP ATP AG"= 1.75 kJ/mol AG"=-18.9 kJ/mol 1,3-Bisphosphoglycerate → 3-Phosphoglycerate Normal free energy of the shown step of glycolysis coupled with synthesis of one molecule of ATP is -18.9 kJ/mol. Calculate AG" (in kJ/mol) of dephosphorylation of 1,3-Biphosphoglycerate if AG" of synthesis of ATP from ADP is 1.75.
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
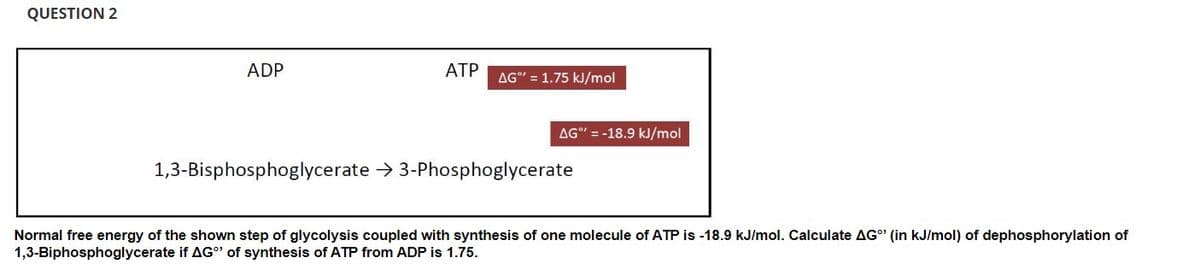
Transcribed Image Text:QUESTION 2
ADP
AG"=-18.9 kJ/mol
1,3-Bisphosphoglycerate → 3-Phosphoglycerate
Normal free energy of the shown step of glycolysis coupled with synthesis of one molecule of ATP is -18.9 kJ/mol. Calculate AG" (in kJ/mol) of dephosphorylation of
1,3-Biphosphoglycerate if AG"¹ of synthesis of ATP from ADP is 1.75.
ATP AG" = 1.75 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON