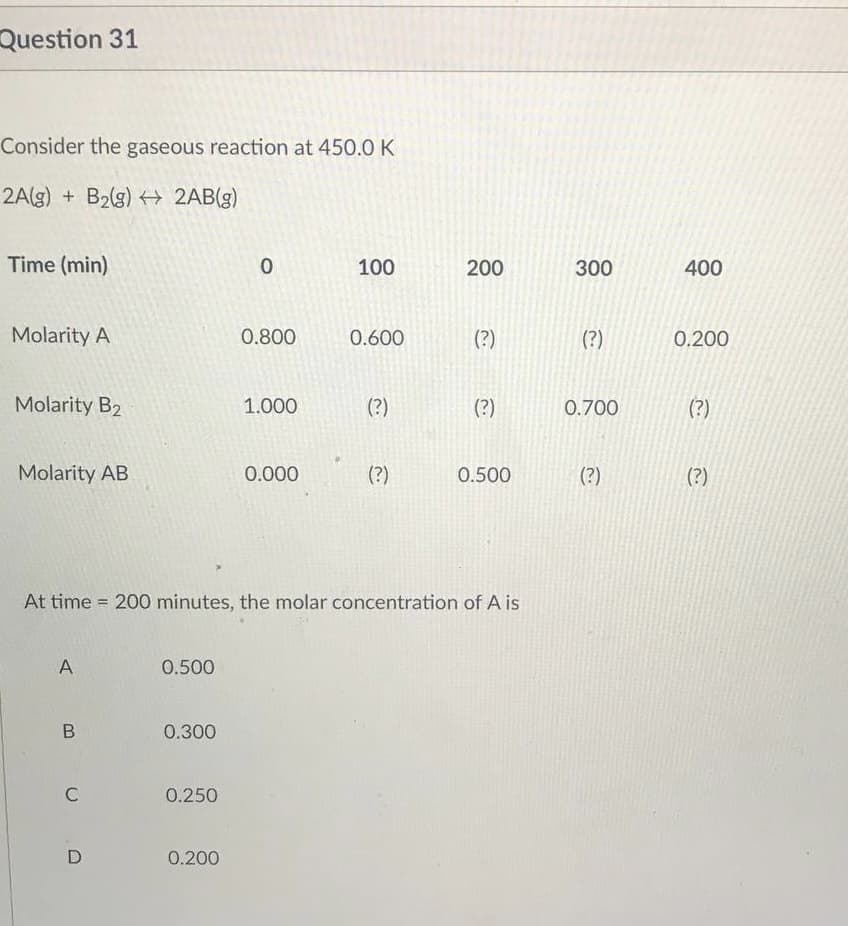
Question 31 Consider the gaseous reaction at 450.0 K 2A(g) + B₂(g) → 2AB(g) Time (min) 0 100 200 Molarity A 0.800 0.600 (?) Molarity B₂ 1.000 (?) (?) Molarity AB 0.000 (?) 0.500 At time 200 minutes, the molar concentration of A is A 0.500 0.300 0.250 0.200 B C D 300 (?) 0.700 (?) 400 0.200 (?) (?)
Q: 2
A: Requirement from Question Five retrosynthetic ways for the cyclo-propane ring. Simmons-smith…
Q: in the blank. H X blank 1 OCH3 رام H₂C-S CI blank 2 OH CH3 H NH H3C blank 3
A:
Q: Organic chemistry, Q: Explain to thoroughly (pretend i'm completely new to the subject) with text…
A: These reactions occurs due to electropositive carbonyl carbon .
Q: Which of the following compound has the highest negative heat of combustion? A. 2-methylheptane…
A:
Q: of a proton in a slow, rate-limiting step gives an anion which then expels the leaving group from…
A: Here we are required to draw the arrow to show the mechanism for the reaction The given reaction…
Q: Draw the sold/dashed wedge structures (sk 4-methylhexane. Label each structure with the proper R/S…
A: Here we have to write structure of all possible stereoisomers of 3,4-dichloro-4-methylhexane and…
Q: Given the following reaction sequence: 1 Br product 4 + 2 Br Br Br Br 7 product 3 3 6 product 2 + 5…
A: We have to predict the Reagent for reaction one.
Q: Calculate [H₂O] of the following polyprotic acid solution: 0.115 M H,CO, (Kat-4.3 × 107, Ka-5.6 x…
A:
Q: 6. Determine the cell voltage for the following cell Fe) + Cd²+ () = Fe²+ (29) + Cd(1) when (a) [Fe]…
A:
Q: ifies the change in internal energy when a molecule is added to a system at constant volume and…
A: Gibb's free energy for closed system can be capable for P-V system work and it uses Helmholtz…
Q: d) CN Br h)
A: -> Every chiral center has a certain configuration either R or S . -> R and S represent two…
Q: 2. Propose a full mechanism using curvy reactions arrows for the following transformation. A
A: The pericyclic reactions are the reaction which involves cyclic transition. It will involve breaking…
Q: Which of the following atoms has the greatest electron affinity (largest positive value)? P Ga Li Br…
A: In this question, we will see which atom have highest positive electron affinity. You can see the…
Q: Provide the correct systematic name for the compound shown here.
A:
Q: H2(g) + 12(g) 2HI(g) The reaction between hydrogen and iodine is first order for both reactants. i)…
A: Given -> H2(g) + I2(g) -----> 2HI(g) -> order with respect to H2(g) is first . -> order…
Q: caculate [H3O+] when pH = 8.42
A: Given that, pH= 8.42 [H3O+]=?
Q: Which keto product would be isolated from the reaction shown? 1. BH3/THF = 2. HO, H2O2. 20 OH OH C A…
A:
Q: In a study of the gas phase decomposition of hydrogen iodide at 700 K HI(g) →½ H₂(g) + ½ I₂(g) the…
A: Given reaction: HI(g) → 12 H2(g) + 12 I2(g) The slope of the plot of 1/[HI] versus time in seconds…
Q: Statement Analysis: Statement 1: AG" is the difference in free energies of the products and…
A:
Q: For the reaction PCl3(g) + Cl₂(g) → PCI5(g) at a particular temperature, Kc = 24.3. Suppose a system…
A:
Q: Write out the SN2 Mechanism for 1-Bromobutane with Nal in acetone, also include transition state,…
A: Alkanes are the organic compounds that contain single bonds between carbon atoms in a compound.
Q: Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene…
A:
Q: 5. a. OCH3 -N =
A: Disconnection approach in organic chemistry: It is an approach for designing organic synthesis that…
Q: The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: E=- In this…
A:
Q: A 1.53 L sample of gas is warmed from 250.0K to a final temperature of 499.0K. Assuming no change in…
A: Given, Initial Volume (V1) = 1.53 L Temperature (T1) = 250.0 K Final Volume (V2) = ? Temperature…
Q: For the reaction 2SO2(g) + O2(g) ⟺ 2SO3(g) at a certain temperature, the equilibrium…
A:
Q: If there is more than one possible site in the molecule/ion, focus on the central or the charged…
A:
Q: 1 Empirical Formula 2 Mass Percent 3 Percent Composition 4 Anhydrous Compound A compound with a…
A: Sorry for the very short answers 1-B 2-J 3-D 4-I 5-A
Q: 4
A: This is an acid base reaction followed by further Alkylation .
Q: The molecules below is Testosterone, which is the male sex hormone. OH CH3 CH3 Which of the…
A: 1. Male sex hormone testosterone has one carbonyl (C=O) group is present as its functionality. The…
Q: Provide the correct common name for the compound shown here. ZI
A:
Q: For HCI (g) AHof = -92.3 kJ/mol. What is AHorxn for each of the following processes? H2 (g) + Cl2…
A:
Q: four common types of radioactive decay, differences between them and a detailed comparison,…
A:
Q: In the reaction aA + bB cC + dD where a, b, c, and d are stoichiometric coefficients, which of the…
A:
Q: 1. Which of the following reactions occurs most rapidly at standard conditions? A. 2Fe(s) + O2(g) →…
A: The correct options are as follows: 1. Option D. 2. Option A. 3. Option C.
Q: Butyric acid (HC4H7O2) is a weak acid with the stench of rancid butter. The pH of 0.50 M HC4H7O2 is…
A: Given pH = 2.56 Concentration (c) = 0.50 M Ka = ?
Q: A compound is made up of 19.1% Co, 34.4% CI, and 46.6% O. A 2.0 L sample of this compound had a mass…
A:
Q: uation with
A: There are four types of radioactive decay are α-decay,β-decay,γ-decay and positron emission.
Q: A balloon fi lled with helium gas, He(g), develops a leak. If it originally contained 0.20 mol of…
A:
Q: Chemistry Light of wavelengths shorter than 275 nm can be used to photodissociate the hydrogen…
A:
Q: Question 9 These conditions indicate that chemical reaction is at equilibrium except A the products…
A: Equilibrium is the state in a reversible reaction at which rate of forward reaction becomes equal to…
Q: N₂(g) + 3 F₂(g) → 2 NF, (g) 1 Predict the signs of AHran and A.Sran for the following process. AH 0…
A: Entropy is the degree of Randomness more the Randomness more spontaneous the Reaction will be .
Q: The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: E-- In this…
A:
Q: When 24.5g of NH 3 reacts, the actual yield of H 2O is 26.5g. What is the percent yield? 4NH 3(…
A: The percentage yield is the ratio of actual yield to that of theoretical yield whole multiplied by…
Q: Approximately how much more acidic is acid rain (pH=4.0) than regular rain (pH=5.6)? a 2x b 1.4x c…
A:
Q: HBr (1 equiv) H₂O2 Drawing Atoms, Bonds and Rings Chal Draw or tap a new bond to see suggestion
A:
Q: The energy E of the electron in a hydrogen atom can be calculated from the Bohr formula: In this…
A: Given , E=-Ryn2 Electron transition from n1=9 to n2=7
Q: ic acid, [x] = + 40.3°, hu be incorrect, suggest about the original Structu below COZH но он HO by
A: Introduction : Substances which rotates the plane of polarized light when passed through them are…
Q: belove some reaction mechanism. in wwhich choice shows the necessary to complete the methenism I HBJ…
A: This is SN1 reaction so we will perform the mechanism of SN1 reaction.
Q: FORLAD 4 Consider the redox reaction where Ag+ (aq) is oxidized to Ago (s) (E° 0x = -1.77 v) and…
A:
Step by step
Solved in 2 steps with 2 images

- A flask is charged with 0.110 mol of A and allowed to react to form B according to the following hypothetical gas-phase reaction. A(g) B(g) The following data are collected. times (s) 0 40 80 120 160 moles of A 0.110 0.066 0.042 0.027 0.016 (a) Calculate the number of moles of B at each time in the table.0 s mol40 s mol80 s mol120 s mol160 s mol(b) Calculate the average rate of disappearance of A for each 40 s interval, in units of mol/s.0 - 40 s mol/s40 - 80 s mol/s80 - 120 s mol/s120 - 160 mol/s mol/sFor the reaction 2N2O5(g) → 4NO2(g) + O2(g), the following data were collected. t (minutes) [N2O5] (mol/L) 0 1.24 × 10–2 10. 0.92 × 10–2 20. 0.68 × 10–2 30. 0.50 × 10–2 40. 0.37 × 10–2 50. 0.28 × 10–2 70. 0.15 × 10–2 The concentration N2O5 at 100 min will be approximately Question 17 options: A) 0.10 × 10–2 mol/L B) 0.01 × 10–2 mol/L C) 0.06 × 10–2 mol/L D) 0.03 × 10–2 mol/L E) none of theseA political candidate fighting for a smoke-free environment conducts an experiment in a room containing 1400 cubic feet of air that is initially free of carbon monoxide. Beginning at time t=0, cigarette smoke containing 4% carbon monoxide is introduced into the room at the rate of 0.1 cubic foot perminute, and the well-circulated mixture is allowed to leave the room at the same rate. If carbon monoxide levels in the air reach 0.012%, it is harmful to the human body. Find the time at which this unhealthy level is reached.
- 1. The decomposition of aqueous sucrose to form the isomers glucose and fructose is a common organic reaction, which requires a strong catalyst: C12H22O11(aq) + H2O(l) → 2C6H12O6(aq). The following data were collected during the process: Time (min) [C12H22O11] (mol/L) 0 0.316 39 0.274 80 0.238 140 0.190 210 0.146 (a)If we performed a new trial with an initial concentration of sucrose of 0.400 mol/L, what concentration would remain after 4.0 h has passed? 2. Methyl isomerizes to acetonitrile, CH3NC(g) → CH3CN(g) at 215°C. The following data were collected during the process: Time (sec) [CH3NC] (mol/L) 2000 0.0110 5000 0.0059 8000 0.0031 12000 0.0014 15000 0.0007 2. (a)Assuming the process continues, what concentration of methyl isonitrile would we expect after 5.00 h?For the gas phase decomposition of dinitrogen pentoxide at 335 K 2 N₂05 4 NO2 + O2 the following data have been obtained: [N₂05], M time, s 0.175 0 Submit Answer 9.06x10-2 140 4.69x10-2 280 2.43x10-2 420 The average rate of disappearance of N₂O5 over the time period from t = 280 s to t = 420 s isCalcium oxide, an important ingredient in cement, is produced by decomposing calcium carbonate at high temperature: CaCO3(s) → CaO(s) + CO2(g) In one reaction, 3.9 kg of calcium carbonate is heated at 589 °C in a 20.0-L vessel. The pressure of CO2 is 0.18 bar after 6.5 minutes. question 1. What is the average rate of CO2 production in mol/min during the 6.5-minute interval? question 2. How many moles of calcium carbonate consumed in the 6.5-minute interval?
- What percentage of the initial amount of S02Cl2 2ilk remain after 2.25 hours?For the following reaction 6 experiments have been run and the data collected is in the following table: 2 MnO4-(aq) + 5 H2C2O4 (aq) + 6 H+ (aq) ---> 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l) Experiment [MnO4-], M [H2C2O4], M [H+], M Rate, M/s 1 0.2410 0.3470 0.2690 0.1147 2 0.3260 0.6210 0.2270 0.2776 3 0.5630 0.5740 0.7420 0.4431 4 0.2410 0.3470 0.3840 0.1147 5 0.4140 0.5740 0.5610 0.3258 6 0.3260 0.4930 0.4910 0.2203 a) Find the order of the reaction with respect to H+. _______________ b) Find the order of the reaction with respect to MnO4-. ______________ c) Find the order of the reaction with respect to H2C2O4. _____________ d) What is the overall order of the reaction? ________________________Consider the following reaction and the experimental data in the table below.2 ??(?) + ?2(?) → 2 ??2(?)Experiment [NO] (M) [O2] (M) Initial Rate (M/s) 1 0.10 0.10 0.18 2 0.10 0.20 0.35 3 0.20 0.20 1.42Calculate the rate of the reaction when [NO] = 0.35 M and [O2] = 0.65 M
- The decomposition of H2O2 was studied and the concentration (in moles per liter) as a function of time was determined. a. Create a spreadsheet in Excel with the following columns and values. Calculate values for the natural logarithm of [H2O2] using an appropriate formula. Time H2O2(M) LN [H2O2] 0 0.862 120 0.556 240 0.394 360 0.246 480 0.149 b. Create a graph of [H2O2] versus time. Use the Scatter chart type and use the subtype that connect the points with a line. c. Create a graph of LN(H2O2) versus time. Use the scatter chart type and use the subtype that does not connect the points with a line. Instead, insert a trendline (linear fit) and display the equation of the line on the graph. d. Using the trendline equation, calculate the natural log of concentration (LN[H2O2] corresponding to a time elapsed of 200 seconds. From that value, calculate the molar concentration of H2O2 at 200 secondsIn a study of the gas phase decomposition of sulfuryl chloride at 600 KSO2Cl2(g)SO2(g) + Cl2(g)the concentration of SO2Cl2 was followed as a function of time.It was found that a graph of ln[SO2Cl2] versus time in minutes gave a straight line with a slope of -4.28×10-3 min-1 and a y-intercept of -6.95 . Based on this plot, the reaction is________. (firest, zero, or second) order in SO2Cl2 and the rate constant for the reaction is min-1.QUESTION 10 Measurements taken during the following reaction showed a concentration of carbon monoxide (CO) of 196.9 mol/L at 13.9 min and of 122.7 mol/L at 29.5 min. CO (g) + NO2 (g) --> CO2 (g) + NO (g) Calculate the average rate of the gain of carbon dioxide (CO2) in M/sec. Write the answer in scientific notation with 3 sig.dig.

