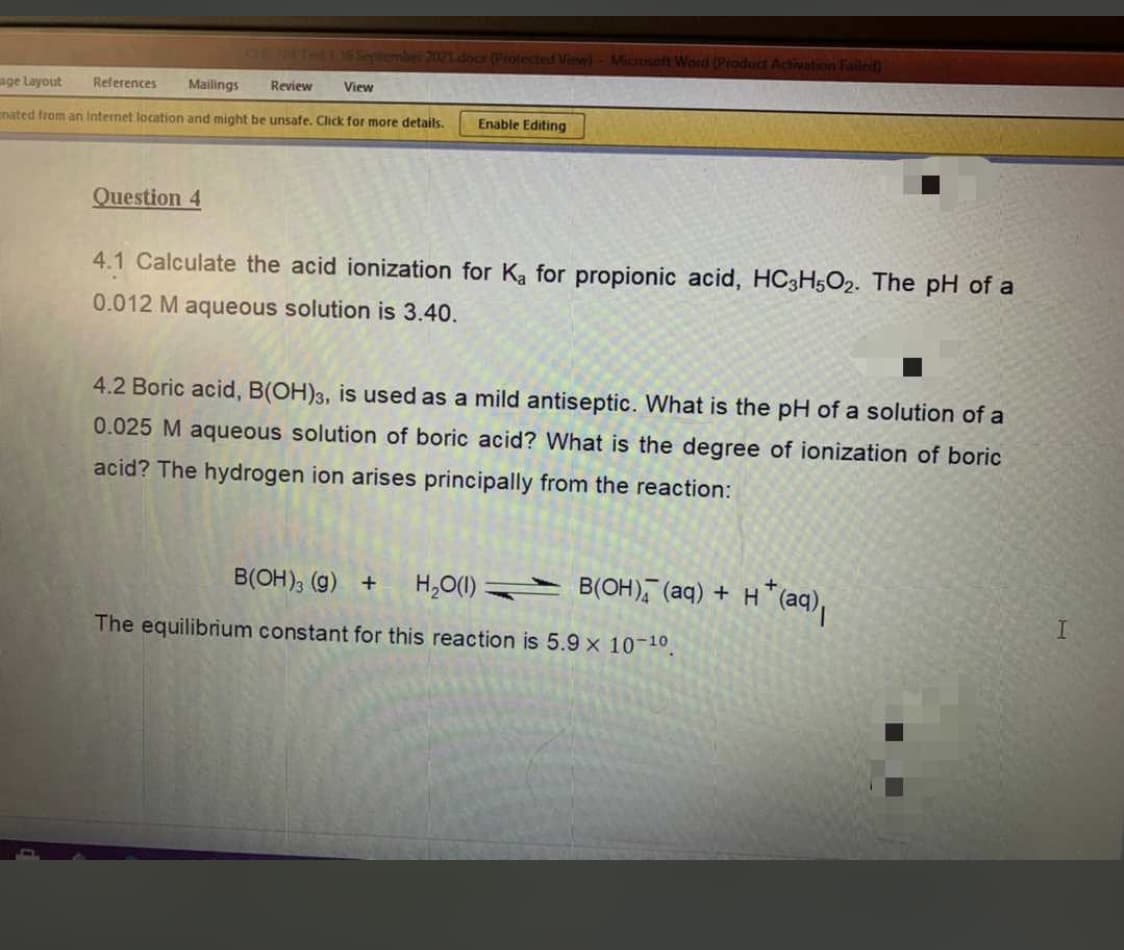
Question 4 4.1 Calculate the acid ionization for Ka for propionic acid, HC3H5O2. The pH of a 0.012 M aqueous solution is 3.40. 4.2 Boric acid, B(OH)3, is used as a mild antiseptic. What is the pH of a solution of a 0.025 M aqueous solution of boric acid? What is the degree of ionization of boric acid? The hydrogen ion arises principally from the reaction: B(OH), (g) + H,O(1) B(OH), (aq) + H *(aq), The equilibrium constant for this reaction is 5.9 x 10-10.
Question 4 4.1 Calculate the acid ionization for Ka for propionic acid, HC3H5O2. The pH of a 0.012 M aqueous solution is 3.40. 4.2 Boric acid, B(OH)3, is used as a mild antiseptic. What is the pH of a solution of a 0.025 M aqueous solution of boric acid? What is the degree of ionization of boric acid? The hydrogen ion arises principally from the reaction: B(OH), (g) + H,O(1) B(OH), (aq) + H *(aq), The equilibrium constant for this reaction is 5.9 x 10-10.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 58P
Related questions
Question
Transcribed Image Text:Septermber 2021Ldoor (Protected View)- Microseft Word (Product Activation Failed
age Layout
References
Mailings
Review
View
nated from an Internet location and might be unsafe, Click for more details.
Enable Editing
Question 4
4.1 Calculate the acid ionization for Ka for propionic acid, HC3H5O2. The pH of a
0.012 M aqueous solution is 3.40.
4.2 Boric acid, B(OH)3, is used as a mild antiseptic. What is the pH of a solution of a
0.025 M aqueous solution of boric acid? What is the degree of ionization of boric
acid? The hydrogen ion arises principally from the reaction:
B(OH); (g) +
H,O(1)
B(OH), (aq) + H
The equilibrium constant for this reaction is 5.9 x 10-10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning