Question 4 of 15 Submit Determine the number of grams of CaHio that are required to completely react to produce 8.70 mol of CO: according to the following combustion reaction: 2 C.Hio(g) + 13 O:(g) - 8 CO:(g) + 10 H:O(g) X STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 2 8.70 8 6.022 x 1023 2.18 0.0374 44.01 4.35 208.00 13 18.02 126 10 58.14 mol O: mol C.Hio gO. g H:O mol H:O g CO. g C.Hio mol CO.
Question 4 of 15 Submit Determine the number of grams of CaHio that are required to completely react to produce 8.70 mol of CO: according to the following combustion reaction: 2 C.Hio(g) + 13 O:(g) - 8 CO:(g) + 10 H:O(g) X STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 2 8.70 8 6.022 x 1023 2.18 0.0374 44.01 4.35 208.00 13 18.02 126 10 58.14 mol O: mol C.Hio gO. g H:O mol H:O g CO. g C.Hio mol CO.
Chapter5: Gases
Section: Chapter Questions
Problem 161IP: In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric...
Related questions
Question
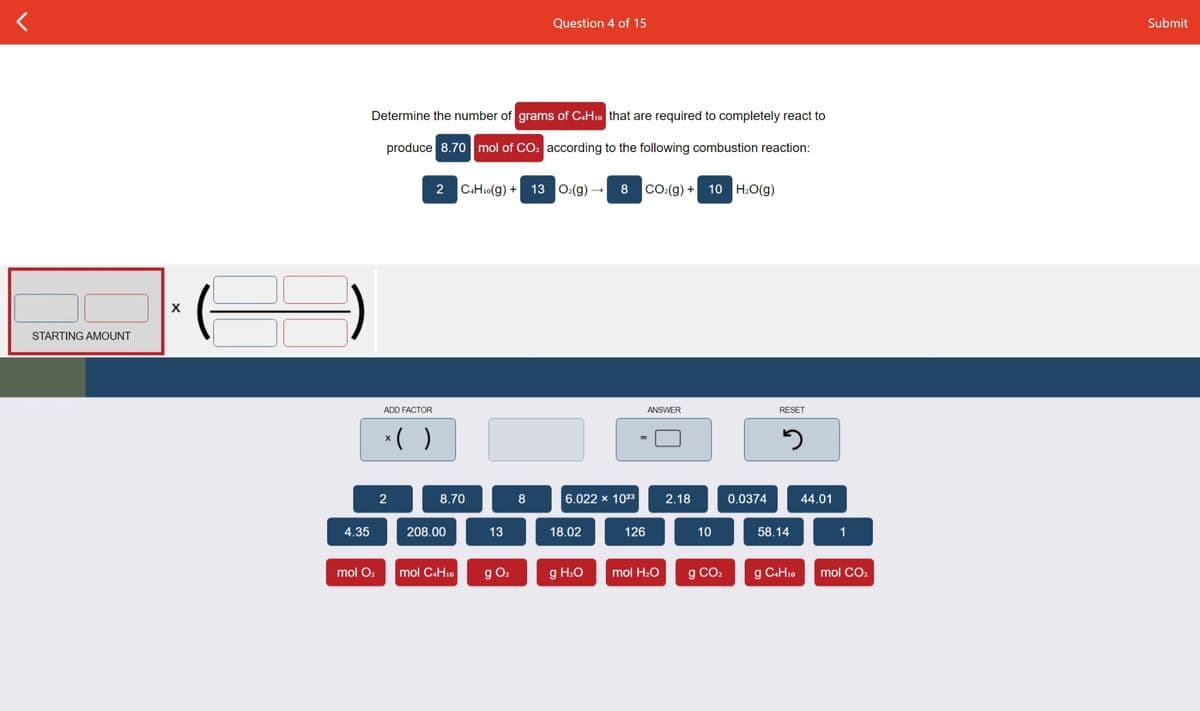
Transcribed Image Text:Question 4 of 15
Submit
Determine the number of grams of CaH10 that are required to completely react to
produce 8.70 mol of CO2 according to the following combustion reaction:
2
C.H10(g) +
13 O:(g)
8
CO:(g) +
10
H:O(g)
STARTING AMOUNT
ADD FACTOR
ANSWER
RESET
*( )
2
8.70
6.022 x 1023
2.18
0.0374
44.01
4.35
208.00
13
18.02
126
10
58.14
1
g O.
g H2O
g CO2
mol CO2
mol O2
mol CAH10
mol H2O
g CH10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning