Question 48 of 50> A0.02837 g sample of gas occupies 10.0 ml. at 293.0 K and L10 atm. Upon further analysis, the compound is found to be 38,734% C and 61.266% F. What is the molecular formula of the compound? molecular formula: Draw the Lewis structure of the compound. Select Draw Rings More Erese
Question 48 of 50> A0.02837 g sample of gas occupies 10.0 ml. at 293.0 K and L10 atm. Upon further analysis, the compound is found to be 38,734% C and 61.266% F. What is the molecular formula of the compound? molecular formula: Draw the Lewis structure of the compound. Select Draw Rings More Erese
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter10: Molecular Structure And Bonding Theories
Section: Chapter Questions
Problem 10.123QE
Related questions
Question
These two images are part of the same question. Please help me with the problem please! I don't quite understand the subject
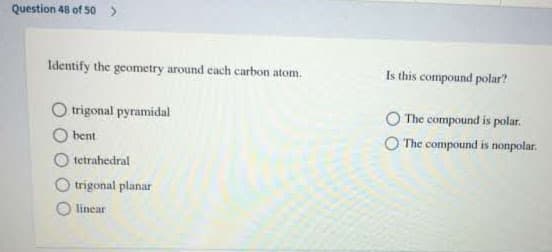
Transcribed Image Text:Question 48 of 50>
Identify the geometry around cach carbon atom.
Is this compound polar?
trigonal pyramidal
The compound is polar.
bent
O The compound is nonpolar.
tetrahedral
trigonal planar
linear
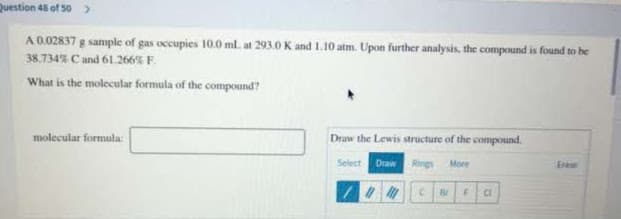
Transcribed Image Text:Question 48 of 50 >
A 0.02837 g sample of gas occupies 10.0 ml. at 293.0 K and L.10 atm. Upon further analysis, the compound is found to be
38.734% C and 61.266% F.
What is the molecular formula of the compound?
molecular formula
Draw the Lewis structure of the compound.
Select
Draw
Rings More
Erase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

