QUESTION 5 Compound A has the formula C8H8. It reacts rapidly with KMNO4 to give CO2 and a carboxylic acid, B (C7H602), but reacts with only 1 molar equivalent of H2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H2 are taken up and hydrocarbon C (C8H16) is produced. What are the structures of A, B, and C. COO CO2H B C
QUESTION 5 Compound A has the formula C8H8. It reacts rapidly with KMNO4 to give CO2 and a carboxylic acid, B (C7H602), but reacts with only 1 molar equivalent of H2 on catalytic hydrogenation over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H2 are taken up and hydrocarbon C (C8H16) is produced. What are the structures of A, B, and C. COO CO2H B C
Chapter30: Orbitals And Organic Chemistry: Pericyclic Reactions
Section30.SE: Something Extra
Problem 36AP
Related questions
Question
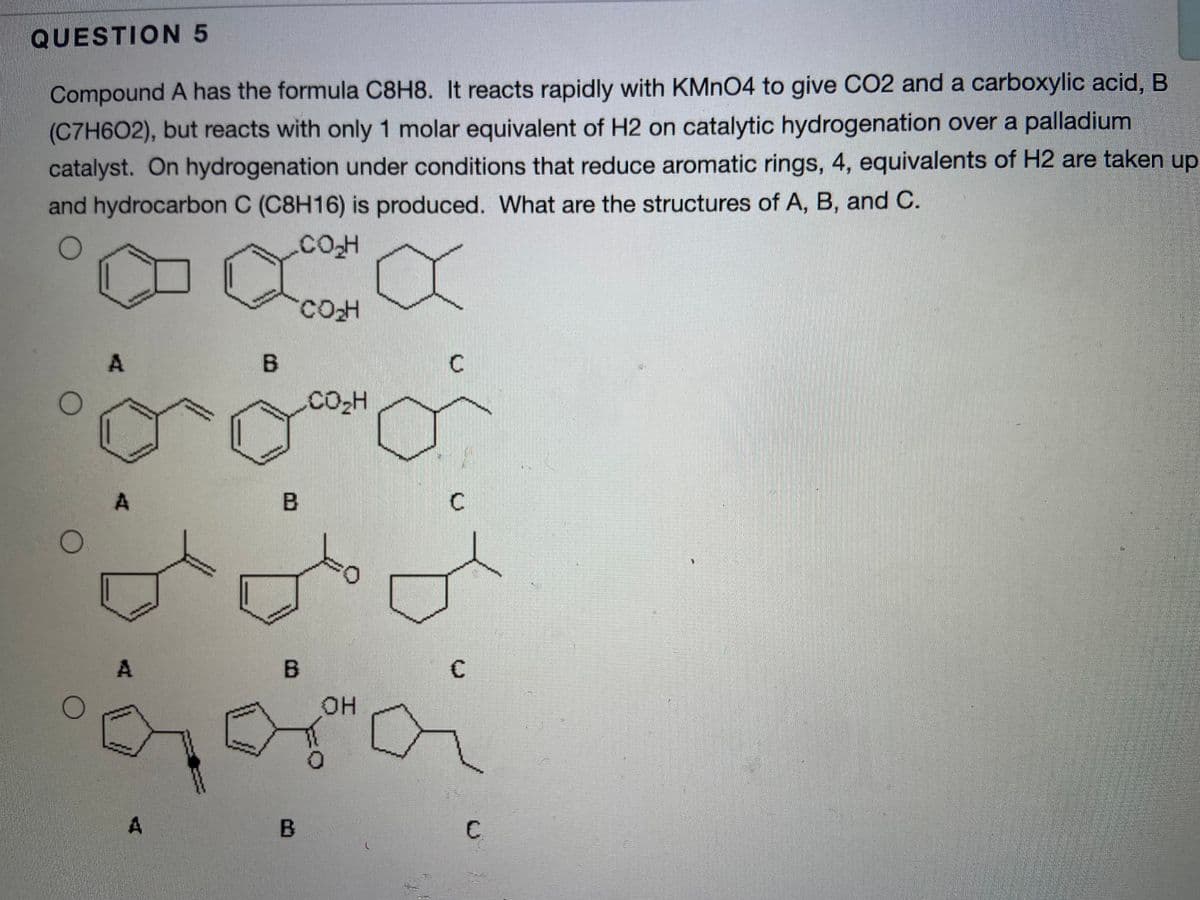
Transcribed Image Text:QUESTION 5
Compound A has the formula C8H8. It reacts rapidly with KMNO4 to give CO2 and a carboxylic acid, B
(C7H6O2), but reacts with only 1 molar equivalent of H2 on catalytic hydrogenation over a palladium
catalyst. On hydrogenation under conditions that reduce aromatic rings, 4, equivalents of H2 are taken up
and hydrocarbon C (C8H16) is produced. What are the structures of A, B, and C.
.COH
A.
B.
CO-H
A
C
он
A
C.
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

