Question 5 Which of the following statement/s is/are TRUE? I. Electrochemistry deals with the physical and chemical processes that involve chemical systems and electricity. II. The underlying concepts and mechanisms in electrochemistry are involved in batteries, supercapacitors, semiconductors, photovoltaics, coulometry, photosynthesis, and many biochemical processes. III. The quantity (Q) of electrical charge is expressed in terms of the Sl unit of coulomb (C). IV. The ability of an electrolyte to allow the flow of electricity is measured in terms of its conductance. V. The molar conductivity of an electrolyte is measured by determining the resistance of the solution contained in a conductivity cell by means of a Wheastone bridge set-up. VI. The plot of the molar conductivity against can be extrapolated to = 0 to yield the molar conductivity at infinite dilution. O I, II, II O All statements are correct. O ,I O , II, IV O , II, II, IV, V O , II, II, IV, VI
Question 5 Which of the following statement/s is/are TRUE? I. Electrochemistry deals with the physical and chemical processes that involve chemical systems and electricity. II. The underlying concepts and mechanisms in electrochemistry are involved in batteries, supercapacitors, semiconductors, photovoltaics, coulometry, photosynthesis, and many biochemical processes. III. The quantity (Q) of electrical charge is expressed in terms of the Sl unit of coulomb (C). IV. The ability of an electrolyte to allow the flow of electricity is measured in terms of its conductance. V. The molar conductivity of an electrolyte is measured by determining the resistance of the solution contained in a conductivity cell by means of a Wheastone bridge set-up. VI. The plot of the molar conductivity against can be extrapolated to = 0 to yield the molar conductivity at infinite dilution. O I, II, II O All statements are correct. O ,I O , II, IV O , II, II, IV, V O , II, II, IV, VI
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 76AP
Related questions
Question
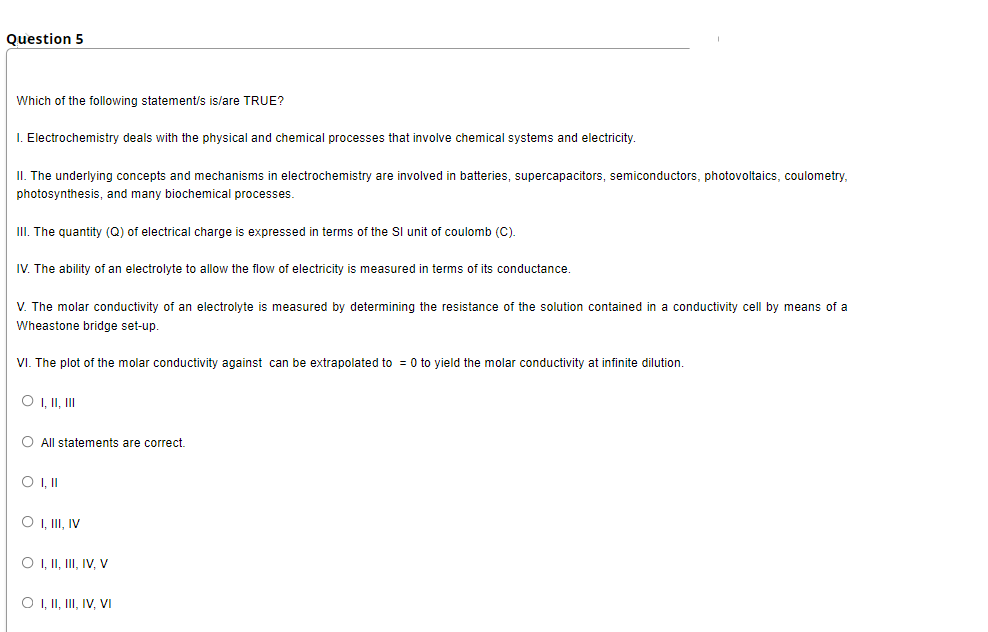
Transcribed Image Text:Question 5
Which of the following statement/s is/are TRUE?
I. Electrochemistry deals with the physical and chemical processes that involve chemical systems and electricity
II. The underlying concepts and mechanisms in electrochemistry are involved in batteries, supercapacitors, semiconductors, photovoltaics, coulometry,
photosynthesis, and many biochemical processes.
III. The quantity (Q) of electrical charge
expressed in terms of the Sl unit of coulomb (C).
IV. The ability of an electrolyte to allow the flow of electricity is measured in terms of its conductance
V. The molar conductivity of an electrolyte is measured by determining the resistance of the solution contained in a conductivity cell by means of a
Wheastone bridge set-up.
VI. The plot of the molar conductivity against can be extrapolated to = 0 to yield the molar conductivity at infinite dilution.
O I, II, II
O All statements are correct.
O I,I
O , II, IV
O , II, III, IV, V
O , II, II, IV, VI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
