Question 6. Inside a rigid container, initially filled with 2.5 kPa CO2 at 383.08K. Then excessive amount of NHCOONH4(S) is added inside and the volume of the solid is negligible Assume the only reaction inside the system is: NH2COONH4(S) 2 NH3 (g) + CO2 (g). After equilibrium, the total pressure of the system is 6.39 kPa. (a) At this temperature if Fe catalyst is added, the NH3 can decompose as: 2NH3 = N2 + 3H2 At 298K, this reaction A, G = -33kJ /mol, A,H = -92.4kJ/mol Assume A, H is constant and there was some other inert gas to keep the pressure at 100 kpa Calculate the partial pressure of N2, H2 and CO2 at equilibrium
Question 6. Inside a rigid container, initially filled with 2.5 kPa CO2 at 383.08K. Then excessive amount of NHCOONH4(S) is added inside and the volume of the solid is negligible Assume the only reaction inside the system is: NH2COONH4(S) 2 NH3 (g) + CO2 (g). After equilibrium, the total pressure of the system is 6.39 kPa. (a) At this temperature if Fe catalyst is added, the NH3 can decompose as: 2NH3 = N2 + 3H2 At 298K, this reaction A, G = -33kJ /mol, A,H = -92.4kJ/mol Assume A, H is constant and there was some other inert gas to keep the pressure at 100 kpa Calculate the partial pressure of N2, H2 and CO2 at equilibrium
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 89AP
Related questions
Question
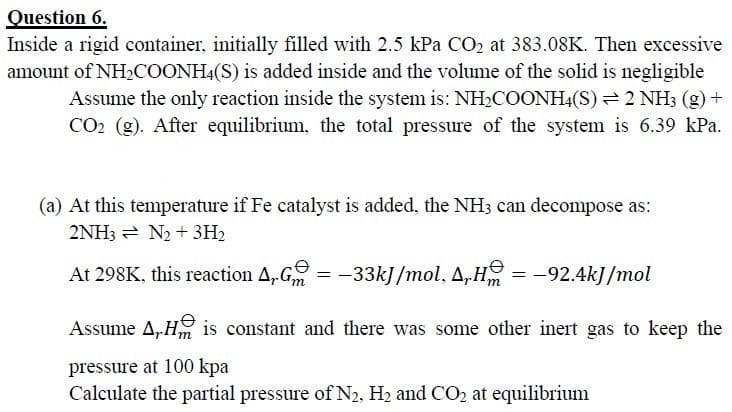
Transcribed Image Text:Question 6.
Inside a rigid container, initially filled with 2.5 kPa CO2 at 383.08K. Then excessive
amount of NH2COONH4(S) is added inside and the volume of the solid is negligible
Assume the only reaction inside the system is: NH2COONH4(S) 2 NH3 (g) +
CO2 (g). After equilibrium, the total pressure of the system is 6.39 kPa.
(a) At this temperature if Fe catalyst is added, the NH3 can decompose as:
2NH3 = N2 + 3H2
At 298K, this reaction A,G
= -33kJ/mol, A,H = -92.4kJ/mol
Assume A,H is constant and there was some other inert gas to keep the
pressure at 100 kpa
Calculate the partial pressure of N2, H2 and CO2 at equilibrium
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning