Chapter9: Covalent Bonding: Orbitals
Section: Chapter Questions
Problem 91CP: A flask containing gaseous N2 is irradiated with 25-nm light. a. Using the following information,...
Related questions
Question

Transcribed Image Text:D
Question 61
Which of the following salts is expected to have the highest melting point?
Cao
OKCI
O NaCl
O CaCl₂
Question 62
Which set of quantum numbers (n, l, m, m,) could represent the last 3d, ground state valence electron irran unknown transition
metal?
(3,2-1, +1/2)
O (3.3.-1.-1/2)
O (4, 2, -2, +1/2)
O (2, 1.0,-1/2)
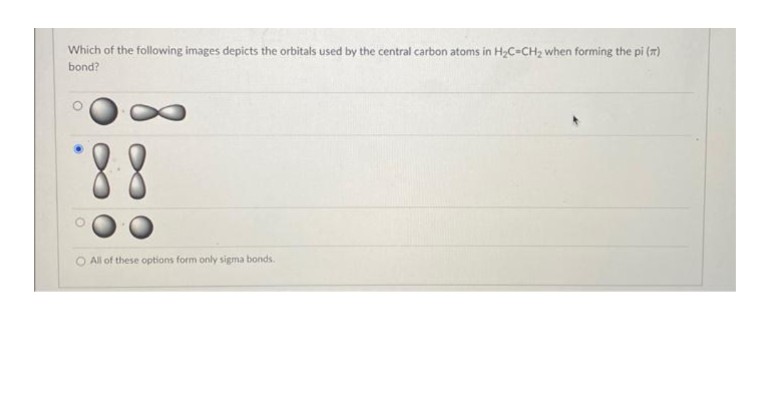
Transcribed Image Text:Which of the following images depicts the orbitals used by the central carbon atoms in H₂C=CH₂ when forming the pi (*)
bond?
*8.8
O All of these options form only sigma bonds.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning