Question 7 The % w/w in a 0.6712-g sample was determined by Volhard titration. After adding 50.00 mL of 0.05619 M AgNO3 and allowing the precipitate to form, the remaining silver was back titrated with 0.05322 M KSCN, requiring 35.14 mL to reach and point. AgNO3+Agl (s) + NO3 Ag+SCN-AgSCN How many milligrams of (MM-126.9 g/mol) are present in the sample? O 119.3 O 0.9400 O 17.76 O 0.6860
Question 7 The % w/w in a 0.6712-g sample was determined by Volhard titration. After adding 50.00 mL of 0.05619 M AgNO3 and allowing the precipitate to form, the remaining silver was back titrated with 0.05322 M KSCN, requiring 35.14 mL to reach and point. AgNO3+Agl (s) + NO3 Ag+SCN-AgSCN How many milligrams of (MM-126.9 g/mol) are present in the sample? O 119.3 O 0.9400 O 17.76 O 0.6860
Chapter17: Complexation And Precipitation Reactions And Titrations
Section: Chapter Questions
Problem 17.35QAP
Related questions
Question
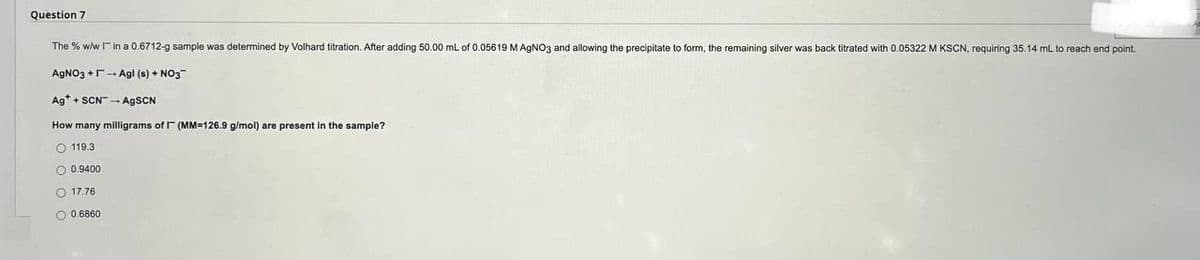
Transcribed Image Text:Question 7
The % w/w I in a 0.6712-g sample was determined by Volhard titration. After adding 50.00 mL of 0.05619 M AgNO3 and allowing the precipitate to form, the remaining silver was back titrated with 0.05322 M KSCN, requiring 35.14 mL to reach end point.
AgNO3 +Agl (s) + NO3
Ag+SCN-AgSCN
How many milligrams of (MM-126.9 g/mol) are present in the sample?
119.3
0.9400
17.76
0.6860
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax