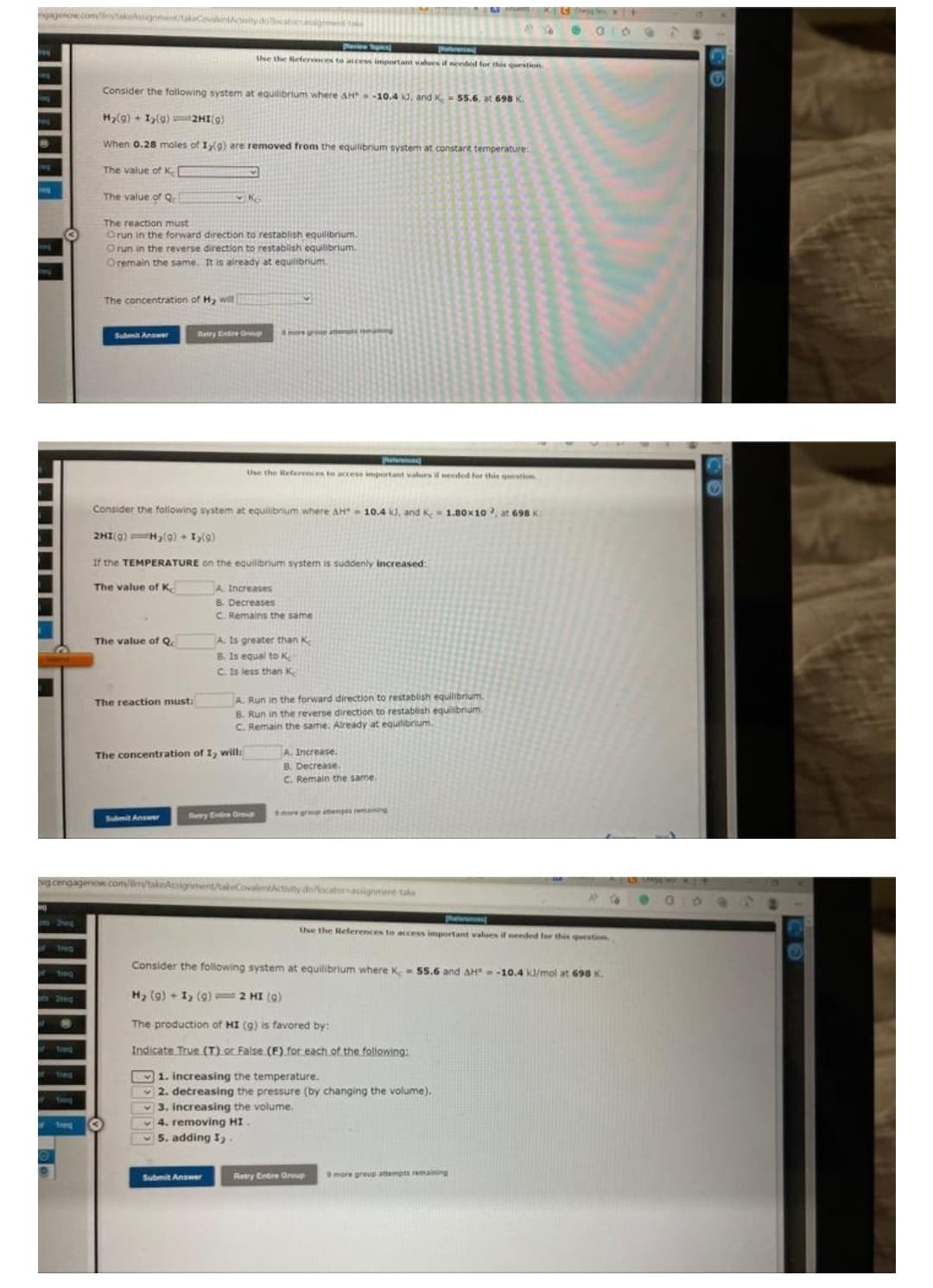
Consider the following system at equilibrium where AH-10.4 id, and X - 55.6, at 698 K. H₂(9)+1₂(9) HI(g) When 0.28 moles of 1,(9) are removed from the equilibrium system at constant temperature: The value of K[ The value of Q 29 The reaction must Orun in the forward direction to restablish equilibrium. Orun in the reverse direction to restablish equilibrium Oremain the same. It is already at equilibrium. The concentration of Hy
Consider the following system at equilibrium where AH-10.4 id, and X - 55.6, at 698 K. H₂(9)+1₂(9) HI(g) When 0.28 moles of 1,(9) are removed from the equilibrium system at constant temperature: The value of K[ The value of Q 29 The reaction must Orun in the forward direction to restablish equilibrium. Orun in the reverse direction to restablish equilibrium Oremain the same. It is already at equilibrium. The concentration of Hy
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter20: Carbohydrates
Section: Chapter Questions
Problem 20.83P
Related questions
Question
Transcribed Image Text:pagenow.com/ry/takeAssignment/takeCovalentActivity d
Paview pics
Paturences
the the References to access important valses if needed for this question
Consider the following system at equilibrium where AH-10.4 kJ, and K = 55.6, at 698 K.
H₂(g) + 1₂(9) 2HI(g)
When 0.28 moles of 1,(9) are removed from the equilibrium system at constant temperature:
The value of K
The value of Qc
K
The reaction must
Orun in the forward direction to restablish equilibrium.
Orun in the reverse direction to restablish equilibrium.
Oremain the same. It is already at equilibrium.
The concentration of Hy will
& more group attempts mean
Submit Answer
Platerences
Use the References to access important values if needed for this question
Consider the following system at equilibrium where AH 10.4 kJ, and K 1.80x102, at 698 K:
2HI(g) H₂(g) + 1₂ (9)
If the TEMPERATURE on the equilibrium system is suddenly increased:
The value of K
A. Increases
B. Decreases
C. Remains the same
The value of Q
A. Is greater than Ke
B. Is equal to Ke
C. Is less than K
The reaction must:
A. Run in the forward direction to restablish equilibrium
B. Run in the reverse direction to restablish equilibrium.
C. Remain the same. Already at equilibrium.
The concentration of I, will:
A. Increase.
B. Decrease.
C. Remain the same.
Submit Answer
Retry Entire Group
more group attempts remaining
vg.cengagenow.com/m/takeAssignment/takeCovalentActivity doocatoraignment take
ng
treq
ps2req
M
freq
▬▬
Retry Entire Gr
(+
0
Patorances
Use the References to access important values if needed for this question
Consider the following system at equilibrium where K= 55.6 and AH = -10.4 kJ/mol at 698 K.
H₂ (9) 1₂ (9) 2 HI(g)
The production of HI (9) is favored by:
Indicate True (T) or False (F) for each of the following:
1. increasing the temperature.
2. decreasing the pressure (by changing the volume).
3. increasing the volume.
4. removing HI
5. adding I₂.
Retry Entire Group
3 more group attempts remaining
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning