Question Help ▼ When radioactive substances decay, the amount remaining will form a geometric sequence when measured over constant intervals of time. The table shows the amount of a radioactive isotope initially and after 2 hours. What are the amounts left after 1 hour, 3 hours, and 4 hours? Hours Elapsed 3 4 Grams of the isotope 1324 326 How much of the isotope is left after 1 hour? grams
Question Help ▼ When radioactive substances decay, the amount remaining will form a geometric sequence when measured over constant intervals of time. The table shows the amount of a radioactive isotope initially and after 2 hours. What are the amounts left after 1 hour, 3 hours, and 4 hours? Hours Elapsed 3 4 Grams of the isotope 1324 326 How much of the isotope is left after 1 hour? grams
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU3: Weather: Phase Changes And Behaviour Of Gases
SectionU3.2: Raindrops Keep Falling: Measuring Liquids
Problem 6E
Related questions
Question
How much of the isotope is left fatter 1 hour? How many grams
Transcribed Image Text:Mathway | Trigonometry Probl x
Desmos Classroom Activities
awasrealize.com/community/dasses/563d20c74b934625bb83249538be8129/assignments/92a5477c1d6d46c0a099f5bd32734c3b/content/5e56ff21-00E
pic 6: MathXL for School: Topic Review Copy 1
6.8.33
Question Help ▼
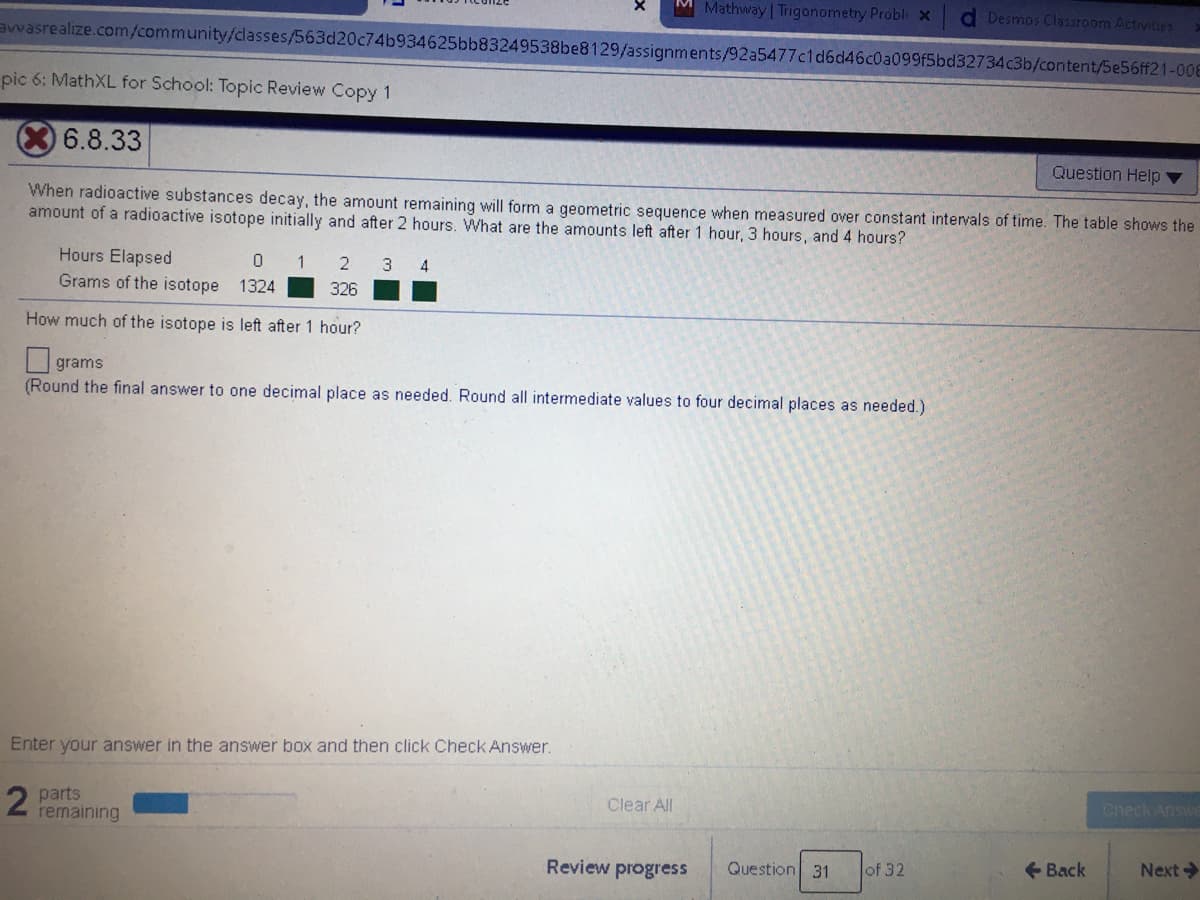
When radioactive substances decay, the amount remaining will form a geometric sequence when measured over constant intervals of time. The table shows the
amount of a radioactive isotope initially and after 2 hours. What are the amounts left after 1 hour, 3 hours, and 4 hours?
Hours Elapsed
0 1
Grams of the isotope 1324
2
3 4
326
How much of the isotope is left after 1 hour?
grams
(Round the final answer to one decimal place as needed. Round all intermediate values to four decimal places as needed.)
Enter your answer in the answer box and then click Check Answer.
Check Answ
Clear All
parts
remaining
Review progress
Question 31
of 32
+ Back
Next
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning