Radioactive Carbon-14 The percentage Pof radioactive carbon- t/5700 14 remaining in a fossil after t years is given by P = 100(÷)*/5700. Suppose a fossil contains 35% of the carbon-14 that the organism %3D contained when it was alive. Estimate the age of the fossil.
Radioactive Carbon-14 The percentage Pof radioactive carbon- t/5700 14 remaining in a fossil after t years is given by P = 100(÷)*/5700. Suppose a fossil contains 35% of the carbon-14 that the organism %3D contained when it was alive. Estimate the age of the fossil.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
ChapterA1: Evaluation Of Analytical Data
Section: Chapter Questions
Problem A1.13QAP
Related questions
Question
answer in the book is 8633 years seems odd
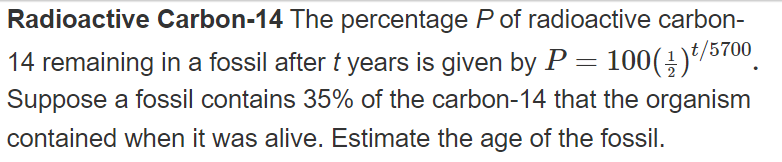
Transcribed Image Text:Radioactive Carbon-14 The percentage P of radioactive carbon-
14 remaining in a fossil after t years is given by P = 100(÷)*/5700.
Suppose a fossil contains 35% of the carbon-14 that the organism
contained when it was alive. Estimate the age of the fossil.

Transcribed Image Text:Exercises 123 O and 124 D: Continuous Compounding Suppose that
P dollars is deposited in a savings account paying 3% interest
compounded continuously. After t years, the account will contain
A (t) = Pe0.03t dollars.
a. Solve A (t) = b for the given values of P and b.
b.
Interpret your results.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

