Radioactive materials are often used in biological studies. A radiation biologist studies the rate of decomposition of a certain substance and obtains the following data: Time (days) 0 4.0 8.0 12.0 16.0 Mass (μg) 26.20 18.13 12.55 8.69 6.01 How many micrograms will remain after 26 days?
Radioactive materials are often used in biological studies. A radiation biologist studies the rate of decomposition of a certain substance and obtains the following data: Time (days) 0 4.0 8.0 12.0 16.0 Mass (μg) 26.20 18.13 12.55 8.69 6.01 How many micrograms will remain after 26 days?
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter16: Reaction Rates
Section: Chapter Questions
Problem 74A
Related questions
Question
Radioactive materials are often used in biological studies. A radiation biologist studies the rate of decomposition of a certain substance and obtains the following data:
| Time (days) | 0 | 4.0 | 8.0 | 12.0 | 16.0 |
| Mass (μg) | 26.20 | 18.13 | 12.55 | 8.69 | 6.01 |
How many micrograms will remain after 26 days?
Expert Solution
Step 1
Radioactive decay follows the first order kinetics. According to the first order reaction :
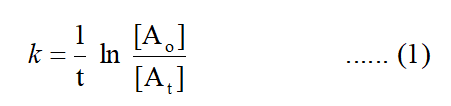
where k is the rate constant, t is the time taken , [Ao] is the initial concentration and [At] is the concentration at time t.
Step 2
Putting the values in equation (1) for determining rate constant k:
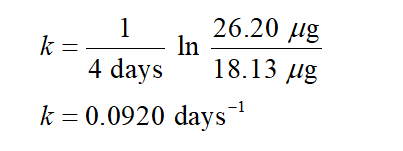
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
