Rank from highest to lowebt first ionization energy. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help element X element Y element Z Lowest first ionization energy Highest first ionization energy O The correct ranking cannot be determined Spoti
Rank from highest to lowebt first ionization energy. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help element X element Y element Z Lowest first ionization energy Highest first ionization energy O The correct ranking cannot be determined Spoti
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter6: Electronic Structure And Periodic Properties Of Elements
Section: Chapter Questions
Problem 72E: Based on their positions in the periodic table, rank the following atoms in order of increasing...
Related questions
Question
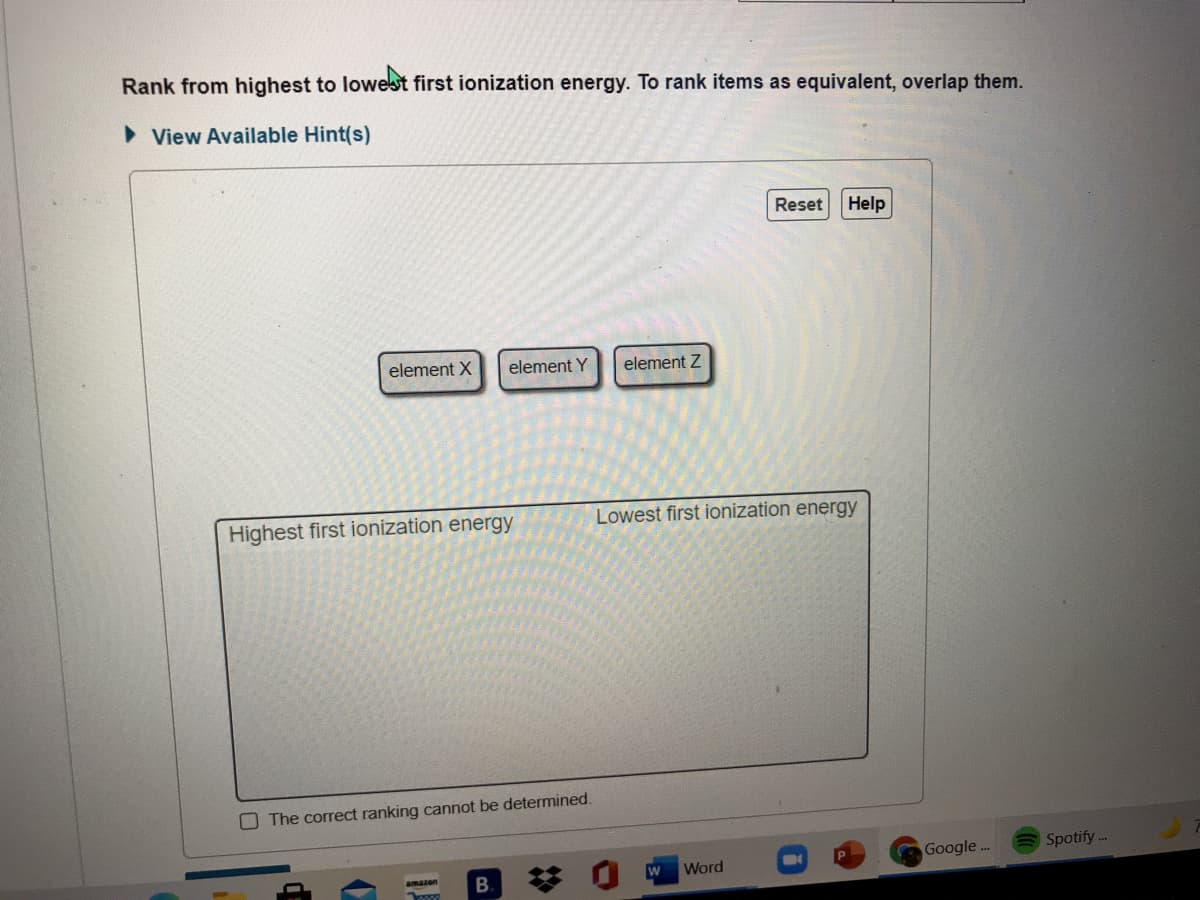
Transcribed Image Text:Rank from highest to lowest first ionization energy. To rank items as equivalent, overlap them.
> View Available Hint(s)
Reset
Help
element X
element Y
element Z
Highest first ionization energy
Lowest first ionization energy
O The correct ranking cannot be determined.
Google .
Spotify -.
Word
amazon

Transcribed Image Text:Part B
For the series of elements X, Y, and Z all in the same period (row), arrange the elements in order of decreasing first ionization energy.
Element
Radius
(pm)
100
Y
180
266
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
