Part A av ( n the alized Based on position in the penodic tabie and oloctron configuration, HIrange thoso oloments in Dder of dacreasing En Rank the elements from highest to lowest ionization energy. To rank items as equivalent, overlap them. • View Available Hint(s) o the alion sing Reset Help
Part A av ( n the alized Based on position in the penodic tabie and oloctron configuration, HIrange thoso oloments in Dder of dacreasing En Rank the elements from highest to lowest ionization energy. To rank items as equivalent, overlap them. • View Available Hint(s) o the alion sing Reset Help
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 55AP: The outermost electron in an alkali-metal atom is sometimes described as resembling an electron in...
Related questions
Question
Transcribed Image Text:michael
CHM 1045
(Post Lecture Homework Chapter 08
lonization Energy
I Review | Constants Pe
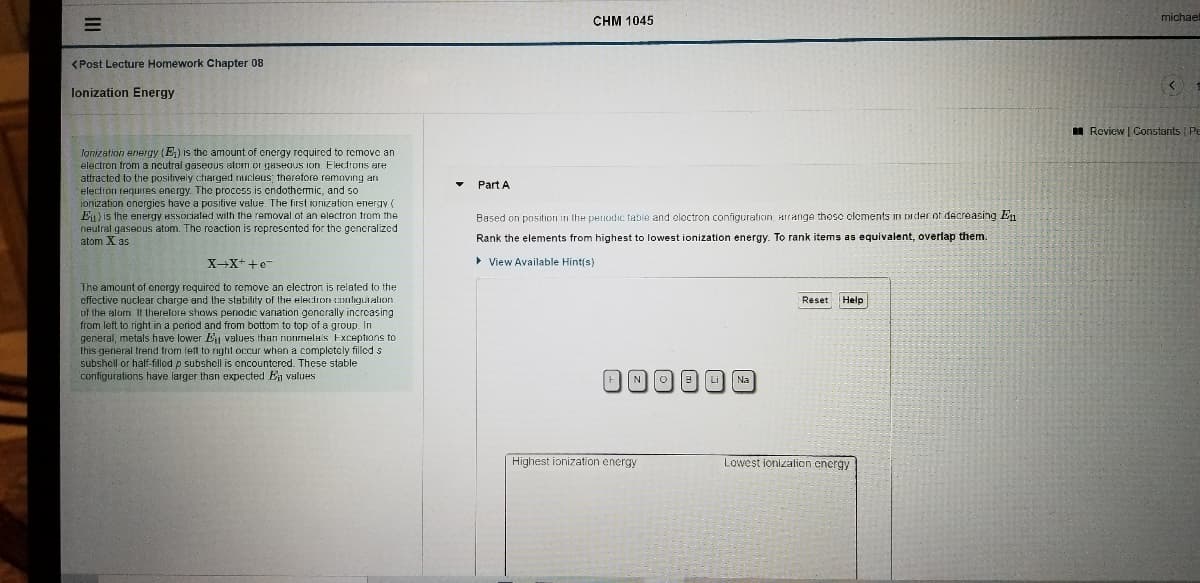
lonization energy (E) is the amount of cnergy required to remove an
electron from a neutral gaseous alom or gaseous ion. Electrons are
attracted to the positively charged nucleus; therefore removing an
Part A
electron requires energy. The process is cndothemic, and so
ionization encrgies have a positive value. The first ionization energy (
Eu) is the energy Hssociated with the removal of an electron trom the
neutral gasecus atom. The reaction is represented for the generalized
atom X as
Based on position in the periodic table and electron configuration, Hirange thesc clements
ader of decreasing En
Rank the elements from highest to lowest ionization energy. To rank items as equivalent, overlap them.
X-X++e
• View Available Hint(s)
The amount of energy required to remove an electron is related to the
effective nuclear charge and the stability of the electron cmligutation
of the atom It therefore shows penodic variation generally increasing
from left to right in a period and from bottom to top of a group. In
general, metals have lower E values ihan nonrmela's Exceptions to
this general trend from left to right occur when a completely filled s
subshell or half-filled p subshell is encountered. These stable
configurations have larger than expected En values
Reset
Help
FNO Li
Na
High
ionization energy
Lowest ionizalion encrgy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning