Rank the following elements in order of decreasing atomic radius. Rank from largest to smallest radius. To rank items as equivalent, overlap them. > View Available Hint(s) Reset Help Be N. B
Rank the following elements in order of decreasing atomic radius. Rank from largest to smallest radius. To rank items as equivalent, overlap them. > View Available Hint(s) Reset Help Be N. B
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter3: Atomic Shells And Classical Models Of Chemical Bonding
Section: Chapter Questions
Problem 1P: Before the element scandium was discovered in 1879, it was known as “eka-boron.” Predict the...
Related questions
Question
100%

Transcribed Image Text:Atomic Radii and Effective Nuclear Charge
The atomic radius of an element can be predicted based on its
periodic properties. Atomic radii increase going down a group in the
periodic table, because successively larger valence-shell orbitals are
occupied by electrons. Atomic radii generally decrease moving from
left to right across a period because the effective nuclear charge
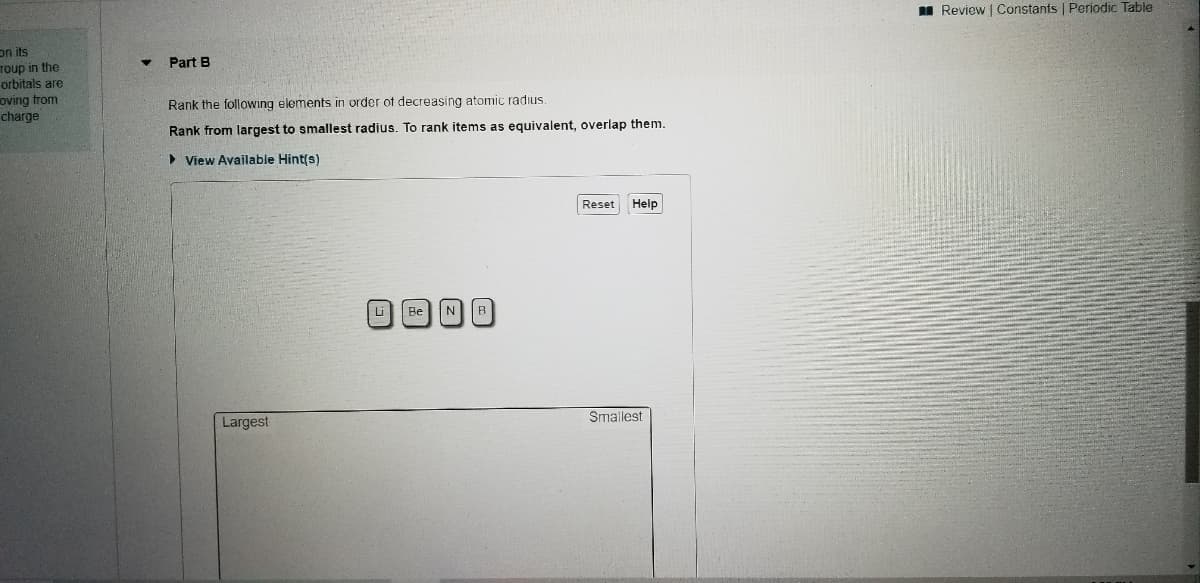
Part B
Rank the following eleme
increases.
Rank from largest to sm
> View Available Hint(s
Transcribed Image Text:I Review | Constants | Periodic Table
on its
roup in the
orbitals are
oving trom
charge
Part B
Rank the following elements in order of decreasing atomic radius.
Rank from largest to smallest radius. To rank items as equivalent, overlap them.
> View Available Hint(s)
Reset
Help
Li
Be
N
B
Smallest
Largest
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning