Rank the following atoms in order of decreasing electronegativity, putting the most electronegative first. Si N I II III IV A) I>IV> II >II B) II> III > IV>I C) III>IV>II >I D) III > II > IV>I Which molecule has the greatest difference in electronegativity (AE) between the two different elements? А) СО В) НS C) NH3 D) H-O
Rank the following atoms in order of decreasing electronegativity, putting the most electronegative first. Si N I II III IV A) I>IV> II >II B) II> III > IV>I C) III>IV>II >I D) III > II > IV>I Which molecule has the greatest difference in electronegativity (AE) between the two different elements? А) СО В) НS C) NH3 D) H-O
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 15STP
Related questions
Question
answer the 2 questions please
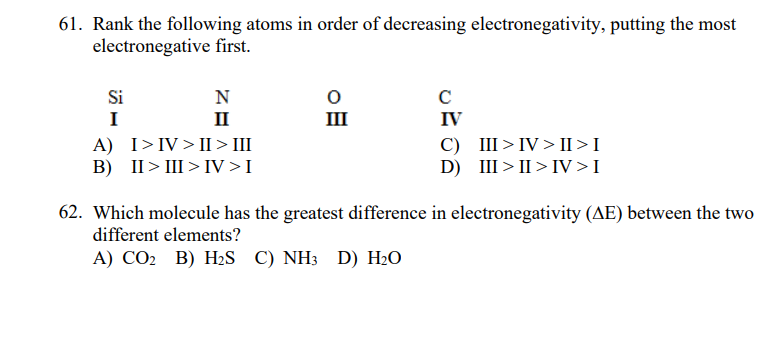
Transcribed Image Text:61. Rank the following atoms in order of decreasing electronegativity, putting the most
electronegative first.
Si
N
C
I
II
III
IV
A) I>IV> II > III
B) II> III > IV>I
C) III>IV > II >I
D) III > II > IV>I
62. Which molecule has the greatest difference in electronegativity (AE) between the two
different elements?
А) СО В) НS C) NH3 D) H-О
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning