atom has the greatest Like other periodic trends of atomic properties, electronegativity trends along the diagonal on the periodic table. Due to poor shielding the, electronegativity value of Select one: a. francium, 5.0 b. helium, 10 O c. hydrogen, 6.0 O d. fluorine, 4.0 Electronegativity differences give a good prediction of bond type. Match the electronegativity difference with bond type. 3.1 Choose... 0.0 Choose... 0.5 Choose... 1.5 Choose...
atom has the greatest Like other periodic trends of atomic properties, electronegativity trends along the diagonal on the periodic table. Due to poor shielding the, electronegativity value of Select one: a. francium, 5.0 b. helium, 10 O c. hydrogen, 6.0 O d. fluorine, 4.0 Electronegativity differences give a good prediction of bond type. Match the electronegativity difference with bond type. 3.1 Choose... 0.0 Choose... 0.5 Choose... 1.5 Choose...
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 15STP
Related questions
Question
For question 2, bond types include...
1. covalent (slightly polar)
2. covalent (pure)
3. ionic
4. polar covalent
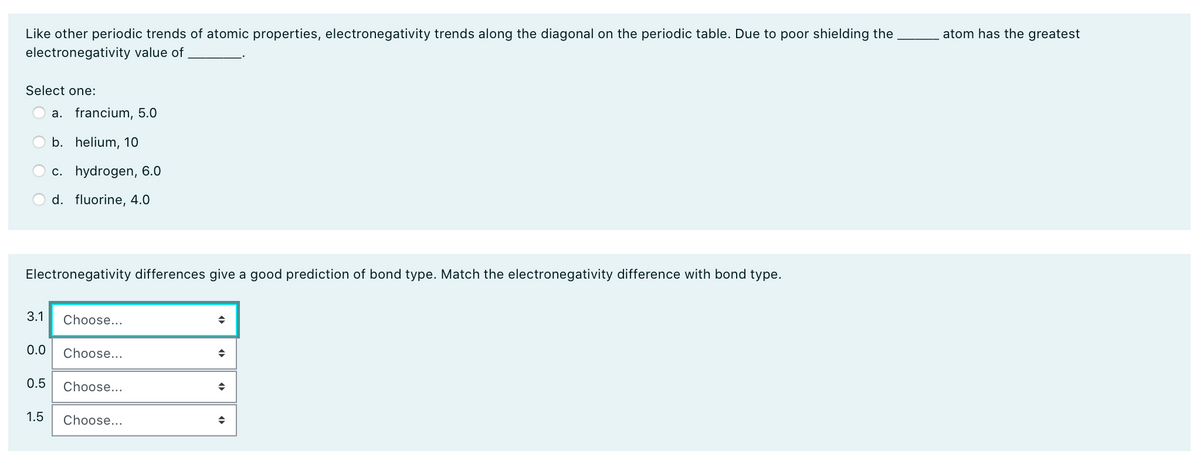
Transcribed Image Text:atom has the greatest
Like other periodic trends of atomic properties, electronegativity trends along the diagonal on the periodic table. Due to poor shielding the
electronegativity value of
Select one:
a. francium, 5.0
b. helium, 10
c. hydrogen, 6.0
d. fluorine, 4.0
Electronegativity differences give a good prediction of bond type. Match the electronegativity difference with bond type.
3.1
Choose...
0.0
Choose...
0.5
Choose...
1.5
Choose...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
