Ranking lonic Solutions: modified MC Chem1B Spr21 Page | 5 D Predict the ranking of the pH for the solutions. 8) Fill in Table ID. Do this before performing the experiment with Beyond Labz Middle column: Identify each solution as one of the following: Strong acid Weak acid Strong base Weak base Acidic salt Neutral salt Basic salt Last column: predict the pH ranking for the following 0.1 M solutions in order of expected increasing pH: rank of 1 will be for the strongest acid and the rank of 14 will be for the strongest base. Table 1D: Predicted Rank (1 most acidic and 14 most basic) Solutions (0.1M) Identification NH,CI NaHCO3 KNO, NH3 H2SO4 NAC2H3O2 (NaAc) HCI
Ranking lonic Solutions: modified MC Chem1B Spr21 Page | 5 D Predict the ranking of the pH for the solutions. 8) Fill in Table ID. Do this before performing the experiment with Beyond Labz Middle column: Identify each solution as one of the following: Strong acid Weak acid Strong base Weak base Acidic salt Neutral salt Basic salt Last column: predict the pH ranking for the following 0.1 M solutions in order of expected increasing pH: rank of 1 will be for the strongest acid and the rank of 14 will be for the strongest base. Table 1D: Predicted Rank (1 most acidic and 14 most basic) Solutions (0.1M) Identification NH,CI NaHCO3 KNO, NH3 H2SO4 NAC2H3O2 (NaAc) HCI
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.9QAP
Related questions
Question
100%
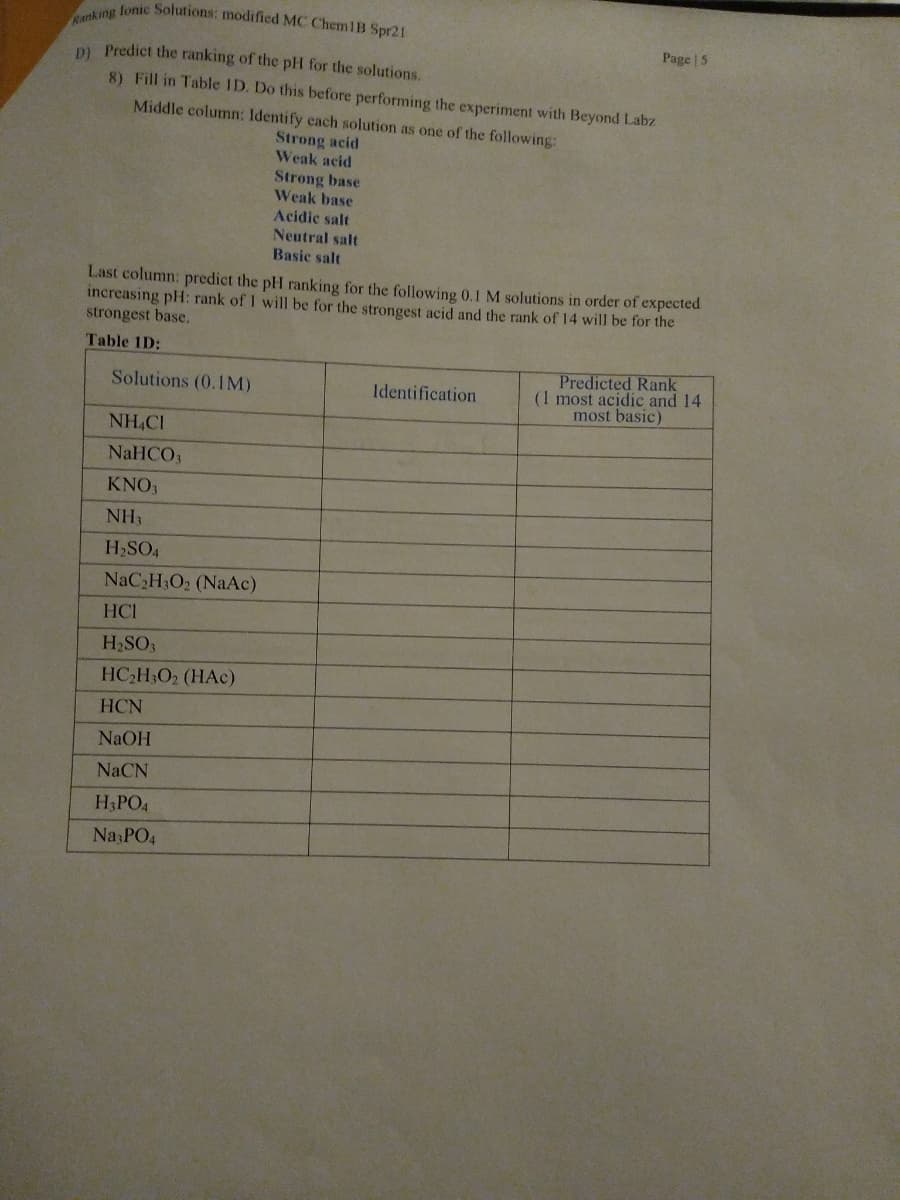
Transcribed Image Text:Ranking lonic Solutions: modified MC ChemlB Spr21
Page | 5
DI Predict the ranking of the pH for the solutions.
8) Fill in Table ID. Do this before performing the experiment with Beyond Labz
Middle column: Identify cach solution as one of the following:
Strong acid
Weak acid
Strong base
Weak base
Acidic salt
Neutral salt
Basic salt
Last column: predict the pH ranking for the following 0.1 M solutions in order of expected
increasing pH: rank of 1 will be for the strongest acid and the rank of 14 will be for the
strongest base.
Table 1D:
Predicted Rank
(1 most acidic and 14
most basic)
Solutions (0.1IM)
Identification
NH,CI
NaHCO3
KNO,
NH3
H2SO4
NaC,H3O2 (NaAc)
HCI
H2SO3
HC2H3O2 (HAc)
HCN
NaOH
NaCN
H3PO4
Na PO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

