1. Prediction of pH and hydrolysis of salts: Aqueous Solutions NH4CI NaCI Na2CO3 CUNO3 NaC2H3O2 NaHSO3 Nat Nat Nat Nat Cation cut co3 2- Anion pH Prediction =7 <7 (<7, >7 or =7) Cation and Anion Hydrolysis Reactions: lon Acidic/Basic/Neutral Hydrolysis Reaction NH4* acidic neu tral CI- Na+ neutral CO32- basic + H40ce) HCO3 caa) + OH"cae) Cut basic Co* + thole) > cU OH +H* NO3 neutral C2H3O2" kg- 1.0xlo HSO3 HSO3 +H20 14) 803caq,) -14 acidic 10 1-SxI07 HISO3 caq) + H20ce) 2 1250s + 0 t caas Kb= ka - 6.7 da kazke
1. Prediction of pH and hydrolysis of salts: Aqueous Solutions NH4CI NaCI Na2CO3 CUNO3 NaC2H3O2 NaHSO3 Nat Nat Nat Nat Cation cut co3 2- Anion pH Prediction =7 <7 (<7, >7 or =7) Cation and Anion Hydrolysis Reactions: lon Acidic/Basic/Neutral Hydrolysis Reaction NH4* acidic neu tral CI- Na+ neutral CO32- basic + H40ce) HCO3 caa) + OH"cae) Cut basic Co* + thole) > cU OH +H* NO3 neutral C2H3O2" kg- 1.0xlo HSO3 HSO3 +H20 14) 803caq,) -14 acidic 10 1-SxI07 HISO3 caq) + H20ce) 2 1250s + 0 t caas Kb= ka - 6.7 da kazke
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.34QAP
Related questions
Question
Need help with the the part highlighted in blue
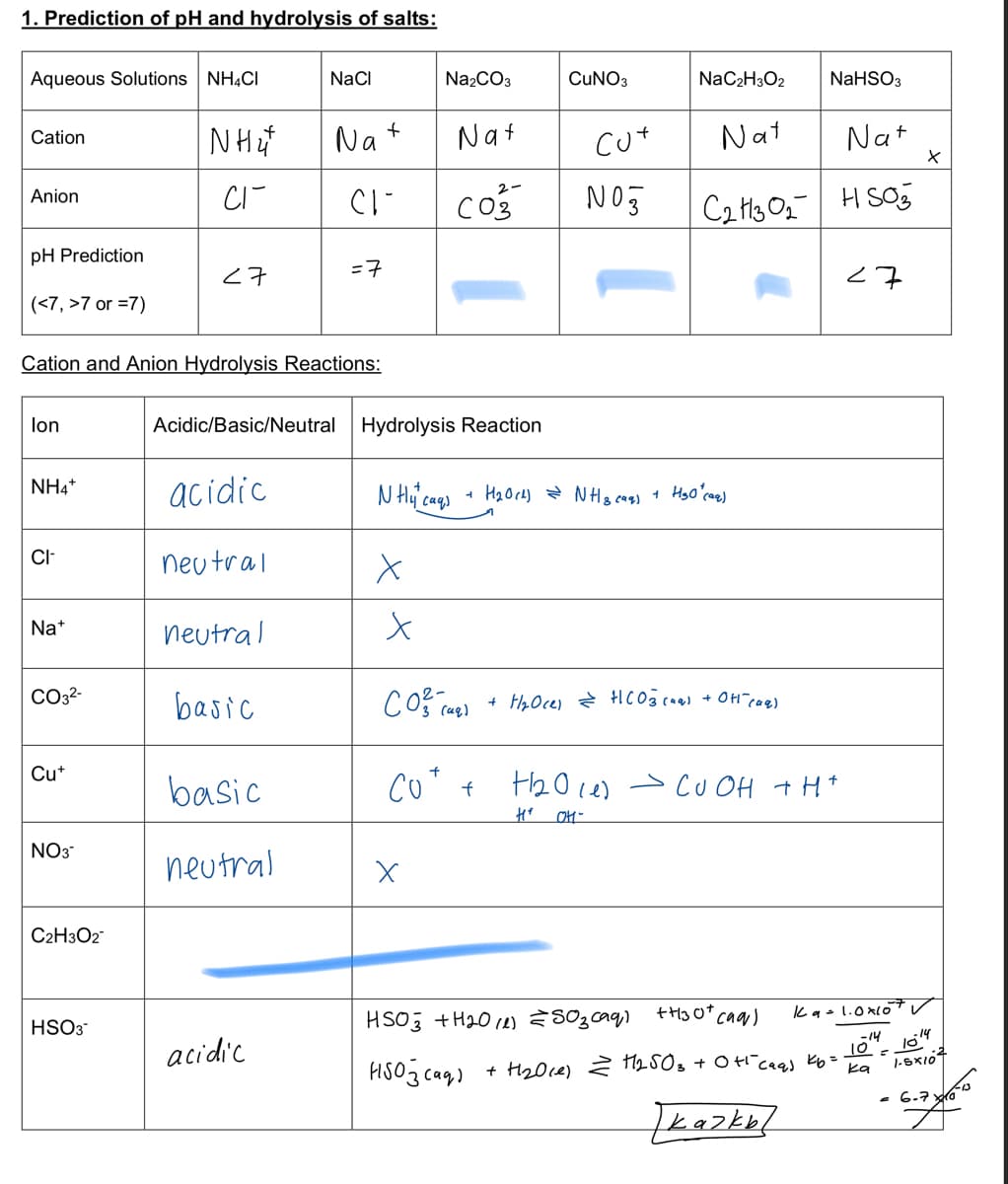
Transcribed Image Text:1. Prediction of pH and hydrolysis of salts:
Aqueous Solutions NH4CI
NaCI
Na2CO3
CUNO3
NaC2H3O2
NaHSO3
Nat
Nat
Nat
Nat
Cation
cut
co3
H SO
2-
Anion
pH Prediction
<7
=7
<7
(<7, >7 or =7)
Cation and Anion Hydrolysis Reactions:
lon
Acidic/Basic/Neutral
Hydrolysis Reaction
NH4*
acidic
neu tral
CI-
Na*
neutral
CO32-
basic
+ HzOce)
Cut
basic
Co* +
> cU OH t H*
NO3
neutral
C2H3O2
kg- 1.0lo
HSO3
HSO3 +H20 14) 803caq,)
-14
acidic
10
1-SxI07
HISO3 cag)
+ H20ce) 2 12503 + 0 t ceas Kb=
ka
- 6.7 da
kazke
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

