Reaction a. HCI (ag) + NaOH (aq) -> NaCl(aq) + h20 (I) B1. NazS2O3 (s) → 2Na* (aq) + S203²- (aq) B2. NazS203 • 5H2O (s) → 2Na* (ag) + S2O3²- (aq) + 5H2O (I) C. Mg (s) + 2H* (aq) → Mg" (aq) + H2 (g) d. Mgo (s) + 2H* (aq) → H20 (I) + Mg2+ (aq) E1. H3PO4 (aq) + NaOH (aq) → NaH,PO4 (aq) + H,O E2. H;PO4 (ag) + 2N2OH (aq) → Na,HPO4 (aq) +2 H20 E3. H;PO4 (aq) + 3NAOH (aq) Na3PO4 (aq) + 3 H,0
Reaction a. HCI (ag) + NaOH (aq) -> NaCl(aq) + h20 (I) B1. NazS2O3 (s) → 2Na* (aq) + S203²- (aq) B2. NazS203 • 5H2O (s) → 2Na* (ag) + S2O3²- (aq) + 5H2O (I) C. Mg (s) + 2H* (aq) → Mg" (aq) + H2 (g) d. Mgo (s) + 2H* (aq) → H20 (I) + Mg2+ (aq) E1. H3PO4 (aq) + NaOH (aq) → NaH,PO4 (aq) + H,O E2. H;PO4 (ag) + 2N2OH (aq) → Na,HPO4 (aq) +2 H20 E3. H;PO4 (aq) + 3NAOH (aq) Na3PO4 (aq) + 3 H,0
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section: Chapter Questions
Problem 111SCQ: Isomers are molecules with the same elemental composition but a different atomic arrangement. Three...
Related questions
Question
100%
Please calculate the literature delta H using the data provided. Please do reactions c-eiii
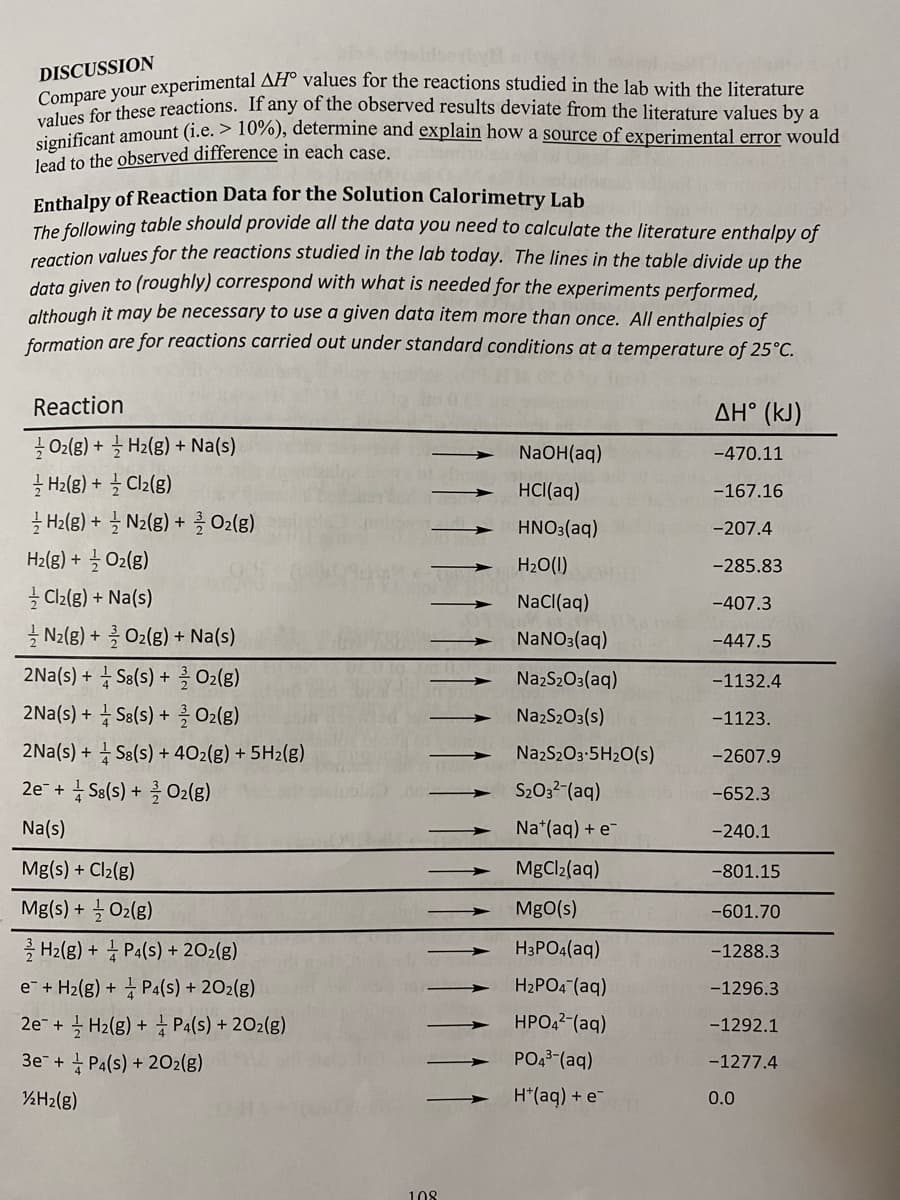
Transcribed Image Text:DISCUSSION
Panere your experimental AH° values for the reactions studied in the lab with the literature
Compare mese reactions. If any of the observed results deviate from the literature values by a
values ont amount (i.e. > 10%), determine and explain how a source of experimental error would
lead to the observed difference in each case
Enthalpy of Reaction Data for the Solution Calorimetry Lab
The following table should provide all the data you need to calculate the literature enthalpy of
reaction values for the reactions studied in the lab today. The lines in the table divide up the
data given to (roughly) correspond with what is needed for the experiments performed,
although it may be necessary to use a given data item more than once. All enthalpies of
formation are for reactions carried out under standard conditions at a temperature of 25°C.
Reaction
ΔΗ° (kJ)
글 olg) + 글 Hzlg) + Na(s)
글 Halg) + 늘 Clzlg)
글 Halg) + 늘 Nz(g) + O2(g)
Hz(g) + 글 O2(g)
Cl2(g) + Na(s)
글 N2(g) + 클 Ox(8) + Na(s)
2Na(s) + + Sa(s) + O2(g)
2Na(s) + 1 Se(s) + 를Oz(g)
NaOH(aq)
-470.11
HCl(aq)
-167.16
HNO3(aq)
-207.4
H20(1)
-285.83
NaCl(aq)
-407.3
NANO3(aq)
-447.5
NazS2O3(aq)
-1132.4
NazS203(s)
-1123.
2Na(s) + S8(s) + 402(g) + 5H2(g)
NazS203-5H20(s)
-2607.9
+ Sa(s) + O2(g)
S203?-(aq)
2e +
-652.3
Na(s)
Na*(aq) + e-
-240.1
Mg(s) + Cl2(g)
MgCl2(aq)
-801.15
Mg(s) + O2(g)
MgO(s)
-601.70
를 H2lg) + 홍 Pa(s) + 202(g)
e + H2(g) + P4(s) + 202(g)
H3PO4(aq)
-1288.3
H2PO4 (aq)
-1296.3
2e- + 글 Hz(g) + Pa(s) + 202(g)
HPO,2-(aq)
-1292.1
3e+ Pa(s) + 20O2(g)
PO43-(aq)
-1277.4
%H2(g)
H*(aq) + e
0.0
108
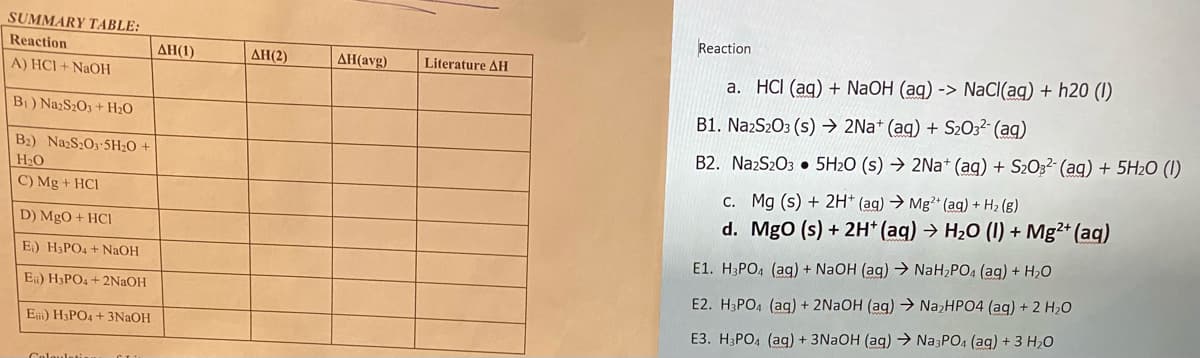
Transcribed Image Text:SUMMARY TABLE:
Reaction
Reaction
ΔΗ (1)
AH(2)
AH(avg)
Literature AH
A) HCI + NaOH
a. HCI (ag) + NaOH (ag) -> NaCI(ag) + h20 (I)
B) NazS2O3 + H20
B1. NażS2O3 (s) → 2Na* (aq) + S2032- (ag)
B2) NazS:O3-5H20 +
B2. NazS2O3 • 5H2O (s) → 2Na* (ag) + S2O3²- (aq) + 5H2O (I)
H2O
C) Mg + HCI
C. Mg (s) + 2H* (ag) → Mg?* (ag) + H2 (g)
d. MgO (s) + 2H* (aq) → H20 (I) + Mg2+ (aq)
D) MgO + HCl
E.) H3PO4 + NaOH
E1. H3PO4 (ag) + NaOH (ag) → NaH,PO4 (ag) + H,O
E) H3PO4 + 2N2OH
E2. H3PO4 (ag) + 2NAOH (aq) → NazHPO4 (ag) + 2 H,0
E) H3PO4 + 3NAOH
E3. H3PO4 (aq) + 3NAOH (aq) → Na3PO4 (aq) +3 H20
Coloulstis. CUU
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning