Reaction B: Sodium Carbonate and Hydrochloric Acid Experimental Data 51.368 g (a) Mass of evaporating dish + watch glass 51.677 g (b) Mass of evaporating dish + watch glass + sodium carbonate 0.309 g (c) Mass of sodium carbonate used 51.671 g (d) Mass of evaporating dish + watch glass + sodium chloride 0.303 g (e) Mass of sodium chloride collected (experimental yield)
Reaction B: Sodium Carbonate and Hydrochloric Acid Experimental Data 51.368 g (a) Mass of evaporating dish + watch glass 51.677 g (b) Mass of evaporating dish + watch glass + sodium carbonate 0.309 g (c) Mass of sodium carbonate used 51.671 g (d) Mass of evaporating dish + watch glass + sodium chloride 0.303 g (e) Mass of sodium chloride collected (experimental yield)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 100CP: he production capacity for acrylonitrile (C3H3N)in the United States is over 2 billion pounds per...
Related questions
Question
Use your data to determine the experimental mole-to-mole ratio between sodium carbonate and sodium chloride. Show your work for each step
a)Convert the mass of sodium carbonate used to moles.
b) Convert the mass of sodium chloride collected to moles.
c) Divide both of your results from the preceding two steps by the lower mole value to determine the simplest mole-to-mole ratio between sodium carbonate and sodium chloride.
Simplest mole ratio before rounding
_____________________moles Na2CO3 : _________________ moles NaCl
Simplest whole number mole ratio after rounding
____________________moles Na2CO3 : _________________moles NaCl
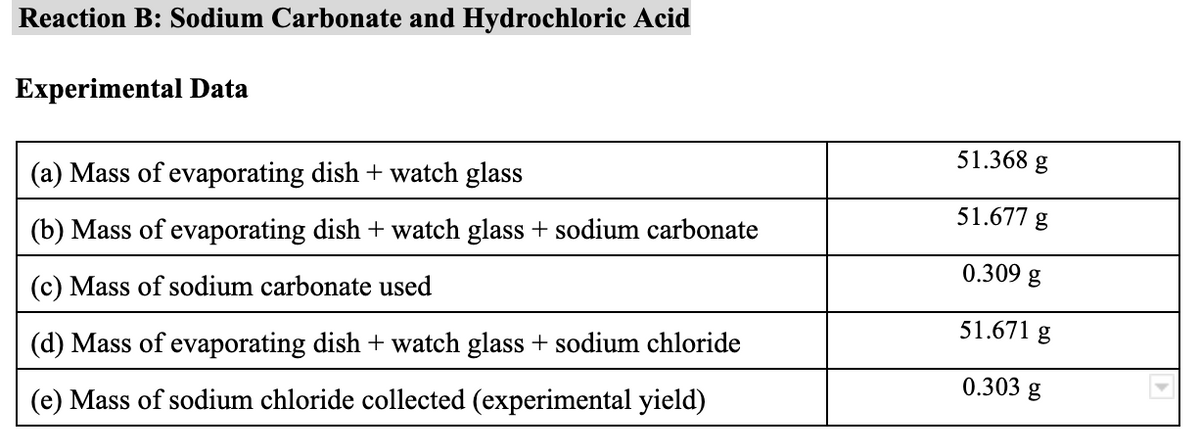
Transcribed Image Text:Reaction B: Sodium Carbonate and Hydrochloric Acid
Experimental Data
51.368 g
(a) Mass of evaporating dish + watch glass
51.677 g
(b) Mass of evaporating dish + watch glass + sodium carbonate
0.309 g
(c) Mass of sodium carbonate used
51.671 g
(d) Mass of evaporating dish + watch glass + sodium chloride
0.303 g
(e) Mass of sodium chloride collected (experimental yield)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning