Read Section 4.3 (Pages 145 - 149) ; Watch KCV 4.3, IWE 4.4. eactions, calculate the mass (in grams) of the product formed when derlined reactant completely reacts. Assume that there is more ne other reactant. Part A 2 K(s) + C2(g) 2 KCI(s) Express your answer using four significant figures. m3= Submit Request Answer Part B 2 K(s) + Br2(1) → 2 KBr(s) Express your answer using four significant figures.
Read Section 4.3 (Pages 145 - 149) ; Watch KCV 4.3, IWE 4.4. eactions, calculate the mass (in grams) of the product formed when derlined reactant completely reacts. Assume that there is more ne other reactant. Part A 2 K(s) + C2(g) 2 KCI(s) Express your answer using four significant figures. m3= Submit Request Answer Part B 2 K(s) + Br2(1) → 2 KBr(s) Express your answer using four significant figures.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter5: Introduction To Chemical Equilibrium
Section: Chapter Questions
Problem 5.40E
Related questions
Question
I am stuck on my chem hw... can anyone help?
Transcribed Image Text:History
Bookmarks
Window
Help
A session.masteringchemistry.com
Sulfuric acid (H2SO4) dissolves aluminum metal ac...
E MasteringChemistry: HW100520
<HW100520
Exercise 4.34- Enhanced- with Feedback
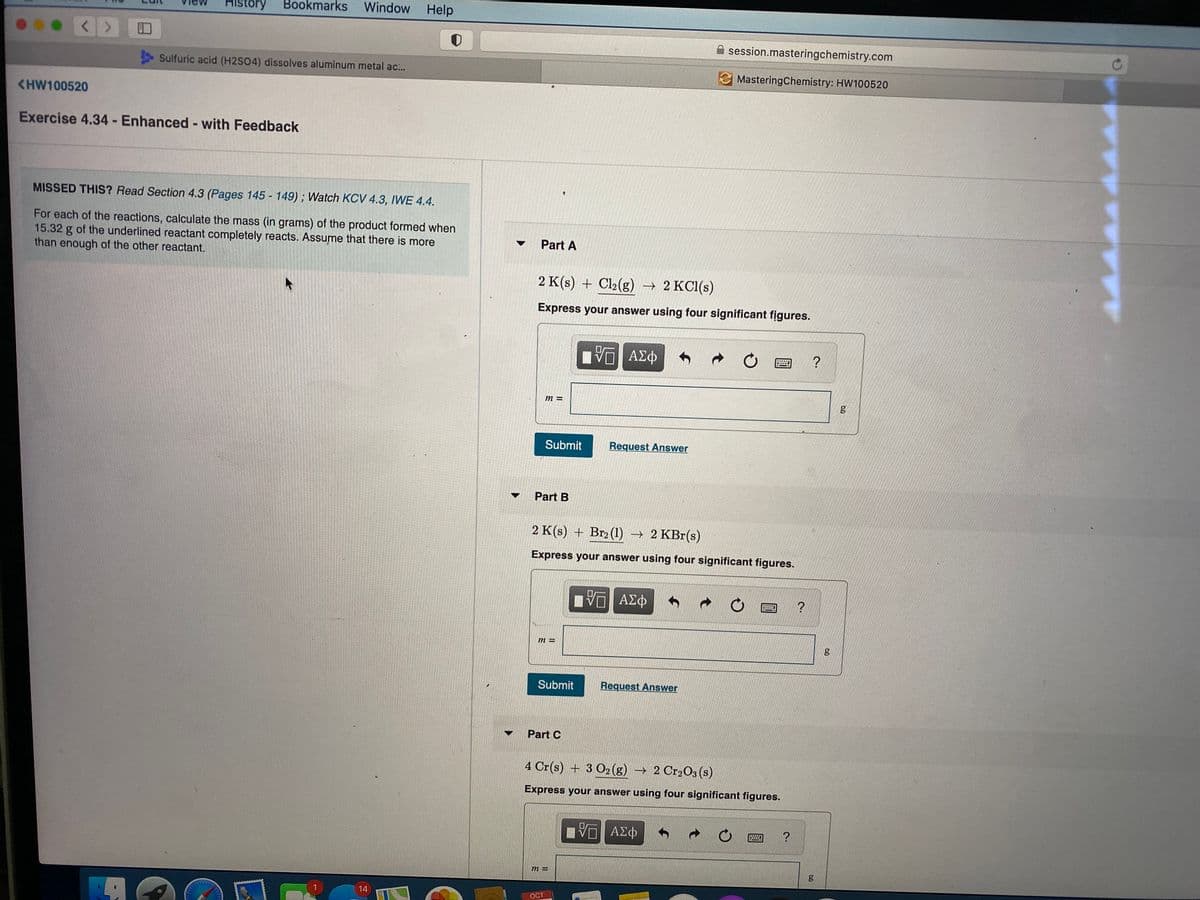
MISSED THIS? Read Section 4.3 (Pages 145 - 149); Watch KCV 4.3, IWE 4.4.
For each of the reactions, calculate the mass (in grams) of the product formed when
15.32 g of the underlined reactant completely reacts. Assume that there is more
than enough of the other reactant.
Part A
2 K(s) + Cl2(g) → 2 KC1(s)
Express your answer using four significant figures.
ΑΣΦ
?
Submit
Request Answer
Part B
2 K(s) + Br2 (1) → 2 KBr(s)
Express your answer using four significant figures.
ανα ΑΣφ
Submit
Request Answer
Part C
4 Cr(s) + 3 O2 (g) → 2 Cr2O3 (s)
Express your answer using four significant figures.
ΑΣΦ
m =
14
OCT
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,