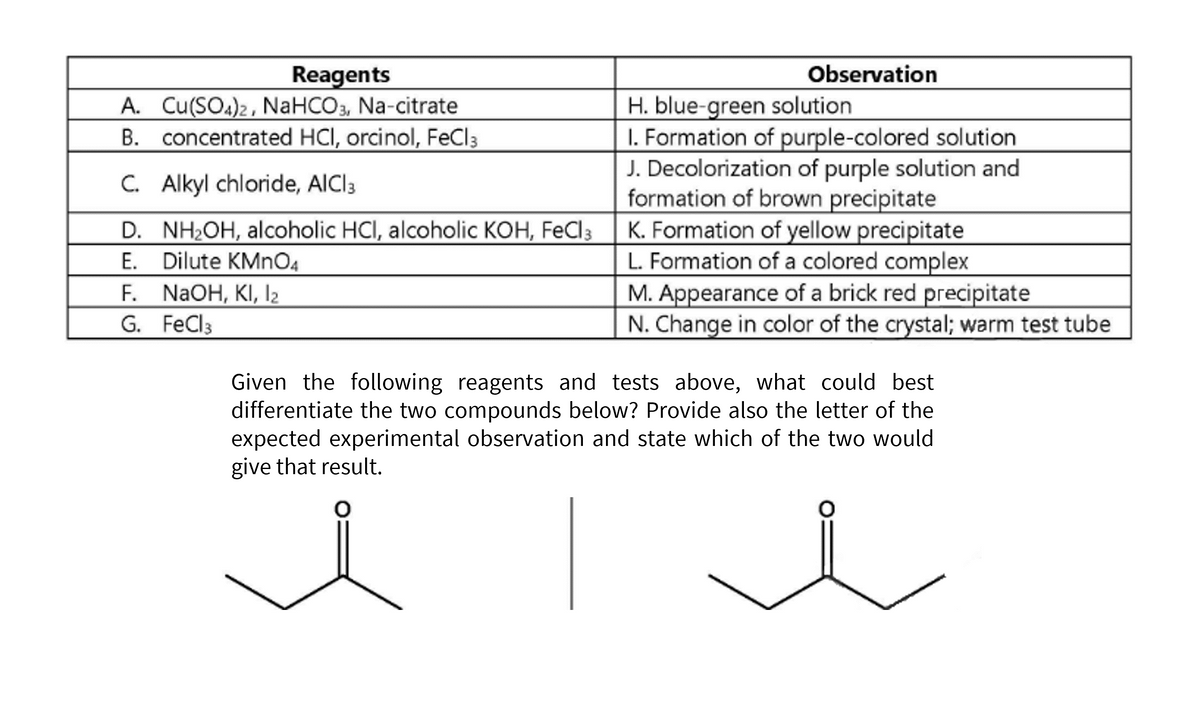
Reagents A. Cu(SO4)2, NaHCO3, Na-citrate B. concentrated HCI, orcinol, FeCl3 C. Alkyl chloride, AlCl3 D. NH₂OH, alcoholic HCI, alcoholic KOH, FeCl3 E. Dilute KMnO4 F. NaOH, KI, 1₂ G. FeCl3 Observation H. blue-green solution 1. Formation of purple-colored solution J. Decolorization of purple solution and formation of brown precipitate K. Formation of yellow precipitate L. Formation of a colored complex M. Appearance of a brick red precipitate N. Change in color of the crystal; warm test tube Given the following reagents and tests above, what could best differentiate the two compounds below? Provide also the letter of the expected experimental observation and state which of the two would give that result.
Reagents A. Cu(SO4)2, NaHCO3, Na-citrate B. concentrated HCI, orcinol, FeCl3 C. Alkyl chloride, AlCl3 D. NH₂OH, alcoholic HCI, alcoholic KOH, FeCl3 E. Dilute KMnO4 F. NaOH, KI, 1₂ G. FeCl3 Observation H. blue-green solution 1. Formation of purple-colored solution J. Decolorization of purple solution and formation of brown precipitate K. Formation of yellow precipitate L. Formation of a colored complex M. Appearance of a brick red precipitate N. Change in color of the crystal; warm test tube Given the following reagents and tests above, what could best differentiate the two compounds below? Provide also the letter of the expected experimental observation and state which of the two would give that result.
Chapter26: Molecular Absorption Spectrometry
Section: Chapter Questions
Problem 26.30QAP
Related questions
Question
Transcribed Image Text:Observation
H. blue-green solution
1. Formation of purple-colored solution
J. Decolorization of purple solution and
formation of brown precipitate
K. Formation of yellow precipitate
L. Formation of a colored complex
M. Appearance of a brick red precipitate
N. Change in color of the crystal; warm test tube
Reagents
A. Cu(SO4)2, NaHCO3, Na-citrate
B. concentrated HCI, orcinol, FeCl3
C. Alkyl chloride, AlCl3
D. NH₂OH, alcoholic HCI, alcoholic KOH, FeCl 3
E.
Dilute KMnO4
F.
NaOH, KI, 12
G. FeCl 3
Given the following reagents and tests above, what could best
differentiate the two compounds below? Provide also the letter of the
expected experimental observation and state which of the two would
give that result.
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
