Redraw the table below and then enter the number of electrons in each quantum level (n) for the following elements. If the quantum level does not contain any electrons, enter a “0” (zero). HINT: For each element in the table, arrange the electrons into the energy level diagram. Next, count the number of electrons in each quantum level (n). For each element in the table, electrons are placed into the energy level diagram. After doing so, you can count the number of electrons in each quantum level (n). Can you help me, please? How can I solve the n=1, n=2, n=3, n=4 for the hydrogen, magnesium, bromine, potassium?
Redraw the table below and then enter the number of electrons in each quantum level (n) for the following elements. If the quantum level does not contain any electrons, enter a “0” (zero). HINT: For each element in the table, arrange the electrons into the energy level diagram. Next, count the number of electrons in each quantum level (n). For each element in the table, electrons are placed into the energy level diagram. After doing so, you can count the number of electrons in each quantum level (n). Can you help me, please? How can I solve the n=1, n=2, n=3, n=4 for the hydrogen, magnesium, bromine, potassium?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter6: Electronic Structure And The Periodic Table
Section: Chapter Questions
Problem 21QAP: What type of electron orbital (i.e., s, p, d, or f) is designated by an electron with quantum...
Related questions
Question
100%
Redraw the table below and then enter the number of electrons in each quantum level (n) for the following elements. If the quantum level does not contain any electrons, enter a “0” (zero). HINT: For each element in the table, arrange the electrons into the energy level diagram. Next, count the number of electrons in each quantum level (n). For each element in the table, electrons are placed into the energy level diagram. After doing so, you can count the number of electrons in each quantum level (n).
Can you help me, please? How can I solve the n=1, n=2, n=3, n=4 for the hydrogen, magnesium, bromine, potassium?
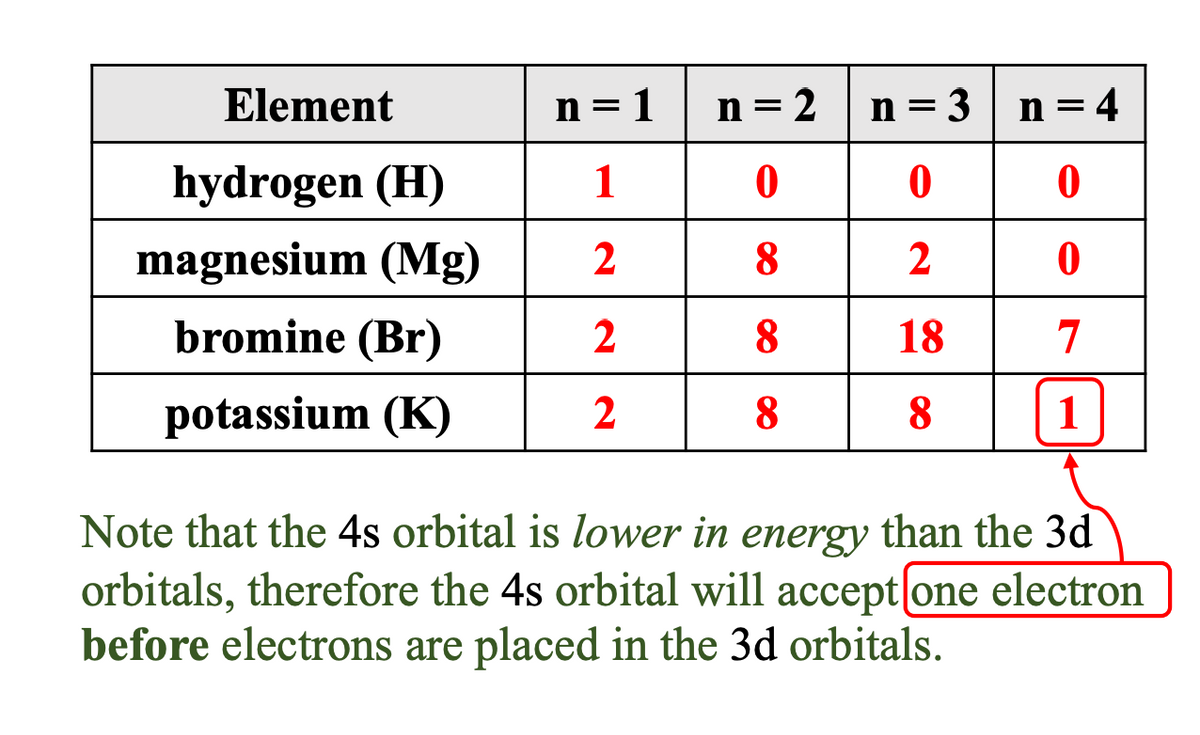
Transcribed Image Text:Element
hydrogen (H)
magnesium (Mg)
bromine (Br)
potassium (K)
n=1
1
2
2
2
n = 2
0
8
8
8
n=3 n = 4
0
0
2
0
18
7
8
1
Note that the 4s orbital is lower in energy than the 3d
orbitals, therefore the 4s orbital will accept [one electron
before electrons are placed in the 3d orbitals.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning