Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
ChapterL: Let's Review
SectionL.4: Problem Solving By Dimensional Analysis
Problem 1RC: 1. A lake has an area of 2.33 × 107 m2. What is this area in square kilometers?
23.3 km2
2.33 × 1010...
Related questions
Question
Chemistry
use data to discuss whichc calculated density is more accurate.
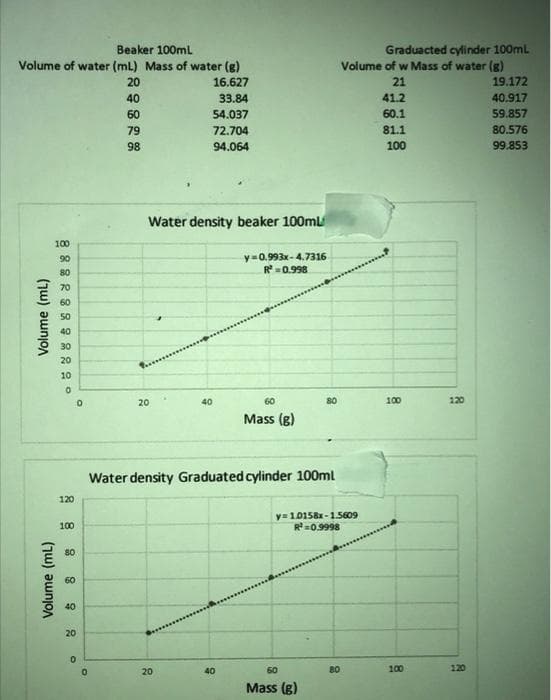
Transcribed Image Text:Beaker 100mL
Volume of water (mL) Mass of water (g)
16.627
33.84
54.037
72.704
94.064
Volume (ml)
Volume (ml)
8822889222°
100
30
120
100
80
60
0
40
20
0
O
20
40
60
79
98
Water density beaker 100ml
20
40
20
y=0.993x-4.7316
R²=0.998
40
60
Mass (g)
Water density Graduated cylinder 100ml
80
Mass (g)
y=10158x-1.5609
R²=0.9998
80
Graduacted cylinder 100mL
Volume of w Mass of water (g)
21
41.2
60.1
81.1
100
100
100
120
120
19.172
40.917
59.857
80.576
99.853
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning