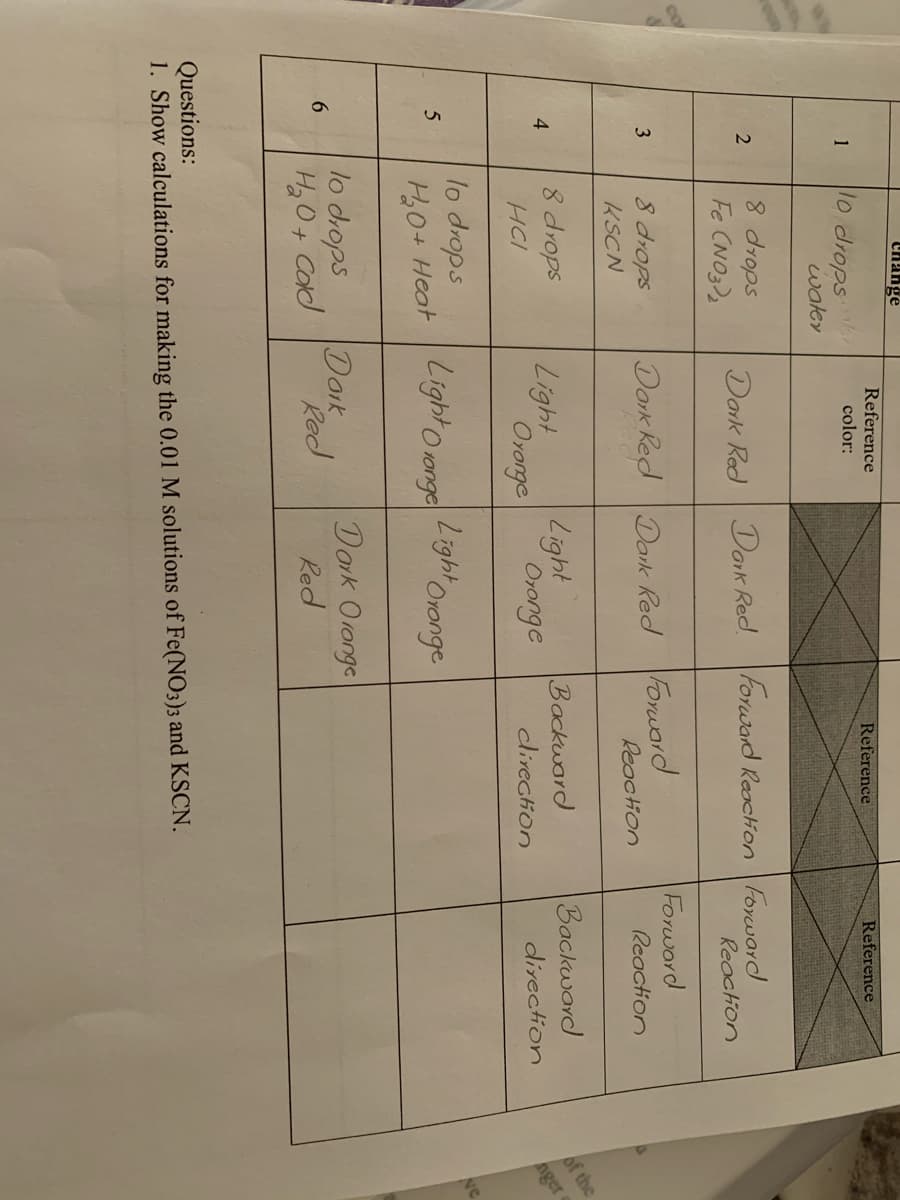
Reference Reference Reference 10 drops waley color: 1 8 drops Fe CNO3)2 Dork Red Dark Red. Forward Reaction Forward Reaction Forword 8 drops KSCN Dork Red Dork Red Forward Reaction 3 Reaction Backward 8 drops Light Backward clivection 4. Ororge Oronge direction HCI lo drops H0+ Heat Light o ronge Light Oronge lo drops Doik Red Dork Oronge Red 6. H2 O+ Cald Questions: 1. Show calculations for making the 0.01 M solutions of Fe(NO3)3 and KSCN.
Reference Reference Reference 10 drops waley color: 1 8 drops Fe CNO3)2 Dork Red Dark Red. Forward Reaction Forward Reaction Forword 8 drops KSCN Dork Red Dork Red Forward Reaction 3 Reaction Backward 8 drops Light Backward clivection 4. Ororge Oronge direction HCI lo drops H0+ Heat Light o ronge Light Oronge lo drops Doik Red Dork Oronge Red 6. H2 O+ Cald Questions: 1. Show calculations for making the 0.01 M solutions of Fe(NO3)3 and KSCN.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 17E: Would the amount of heat absorbed by the dissolution in Example 5.6 appear greater, lesser, or...
Related questions
Question
I need help with my homework
question 5 6 and the question 1
Transcribed Image Text:4,
Change
Reference
10 drops
water
1
color:
Reference
Reference
8 drops
Fe CNO3)2
Dark Red Dark Red. Forward Reacion Foraward
Reaction
Forword
8 drops
KSCN
Dork Red Dank Red Forwarod
Reaction
Reaction
8 drops
Light
Light
Backward
Backward
of the
HCI
Oronge
Oronge
dlirection
direction
nger
lo drops
H0+ Heat
Light Oronge
Light o 1onge
lo drops
HO+ Cad
Doik
Red
Dork Oronge
Red
Questions:
1. Show calculations for making the 0.01 M solutions of Fe(NO3)3 and KSCN.

Transcribed Image Text:When the system contains mostly of reactants, the solution is yellow. When the system shifts towards
products, the color changes to red. By observing the color change, we can see the shift in the equilibrium
between reactants and products.
Addition of hydrochlorie acid provides chloride ions, which produce FeClá (a complex ion) when
combined with iron (III) ions. This is a side reaction that removes the Fe* ions as shown in equation (2). This
disrupts the equilibrium established in the main reaction (1). The main equilibrium will shift back to the
reactants.
Fe* (aq) +
yellow
CF (aq) = FeCla" (aq)
colorless
(2)
colorless
Materials
Equipment small beaker
6 x test tubes
5х
test tube rack
pipettes
2 x 250 mL beakers ice
Chemicals
0.01 M Fe(NO3)3
3M HCI
1M Fe(NO3)3
IM KSCN
0.01 M KSCN
Procedure
1. Make 50 mL of a 0.01 M Fe(NO3)3 and 50 mL of a 0.01 M KSCN from the provided 1.0 M solutions.
2. In a small beaker mix 10 mL of 0.01 M Fe(NO3)3 and 10 mL of 0.01 M KSCN. This is your “stock"
solution.
3. Set up 6 test tubes on a rack. To each test tube add 3 mL of the stock solution you just made.
4. Add 10 drops of water to test tube 1. Record the color of the solution. This is your control sample and you
will compare the colors of all other test tubes to this test tube.
5. Add slowly 8 drops of 1 M Fe(NO3)3 to test tube 2. Be sure to mix the contents by flicking the bottom of the
test tube with the tip of one finger while holding the top of the test tube with your thumb and index finger of
your other hand. Record the color of the solution and compare it to test tube 1.
6. Add slowly 8 drops of 1 M KSCN to test tube 3. Mix as before and record the color you observe and any
change in color.
7. Add slowly, 8 drops of 3 M HCl to test tube 4. Mix as before and record the color observed and any change
in color
8. To test tube 5, add slowly 10 drops of water and place this test tube in a hot water bath (a beaker with hot
water). After 10 minutes, record the color observed any change in color.
9. To test tube 6, add slowly 10 drops of water and place this test tube in a cold water bath (a beaker
containing a mixture of ice and water). After 10 minutes, record the color observed any change in color.
10. Repeat steps 2-9 again. You should have enough stock solution.
11. Disposal: All the solutions must be disposed of in the properly designated waste container.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning