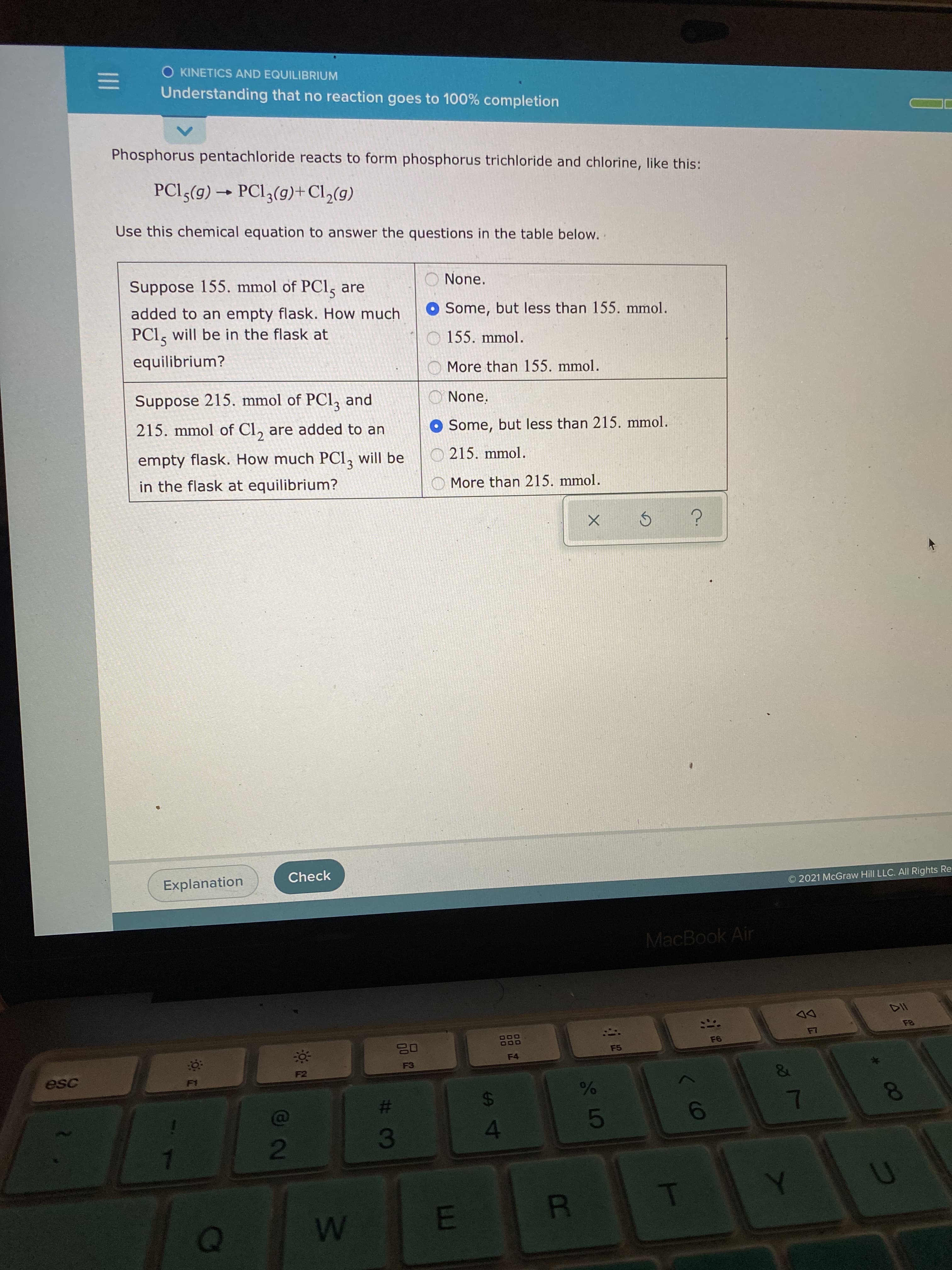
Phosphorus pentachloride reacts to form phosphorus trichloride and chlorine, like this: PCI5(g) → PCI3(g)+Cl,(g) Use this chemical equation to answer the questions in the table below. . O None. Suppose 155. mmol of PCl, are added to an empty flask. How much O Some, but less than 155. mmol. PCI, will be in the flask at 155. mmol. equilibrium? More than 155. mmol. Suppose 215. mmol of PCl, and O None. 215. mmol of Cl, are added to an O Some, but less than 215. mmol. 215. mmol. empty flask. How much PCI, will be in the flask at equilibrium? O More than 215. mmol.
Phosphorus pentachloride reacts to form phosphorus trichloride and chlorine, like this: PCI5(g) → PCI3(g)+Cl,(g) Use this chemical equation to answer the questions in the table below. . O None. Suppose 155. mmol of PCl, are added to an empty flask. How much O Some, but less than 155. mmol. PCI, will be in the flask at 155. mmol. equilibrium? More than 155. mmol. Suppose 215. mmol of PCl, and O None. 215. mmol of Cl, are added to an O Some, but less than 215. mmol. 215. mmol. empty flask. How much PCI, will be in the flask at equilibrium? O More than 215. mmol.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 86QAP
Related questions
Question
Transcribed Image Text:%24
II
O KINETICS AND EQUILIBRIUM
Understanding that no reaction goes to 100% completion
Phosphorus pentachloride reacts to form phosphorus trichloride and chlorine, like this:
PCI5(g) → PCI3(g)+Cl,(g)
Use this chemical equation to answer the questions in the table below.
None.
Suppose 155. mmol of PCl, are
O Some, but less than 155. mmol.
added to an empty flask. How much
PCl, will be in the flask at
155. mmol.
equilibrium?
More than 155. mmol.
Suppose 215. mmol of PCl, and
O None.
215. mmol of Cl, are added to an
O Some, but less than 215. mmol.
empty flask. How much PCl, will be
O 215. mmol.
in the flask at equilibrium?
O More than 215. mmol.
Check
Explanation
O 2021 McGraw Hill LLC. All Rights Re
MacBook Air
DD
F5
F4
esc
%23
4.
3.
FI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning