When comparing the first ionization energies of elements vithin the same period, it is noted that boron first ionization energy than beryllium. This is due to the greater shielding has a of the electrons from the subshell to an area closer to the nucleus, which is not possible for boron the electron present in the atom. As a result, this electron is penetration shielded, making it to remove. 2p harder beryllium smaller ineffectively easier effectively 25
When comparing the first ionization energies of elements vithin the same period, it is noted that boron first ionization energy than beryllium. This is due to the greater shielding has a of the electrons from the subshell to an area closer to the nucleus, which is not possible for boron the electron present in the atom. As a result, this electron is penetration shielded, making it to remove. 2p harder beryllium smaller ineffectively easier effectively 25
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 126CP
Related questions
Question
Transcribed Image Text:Reset Help
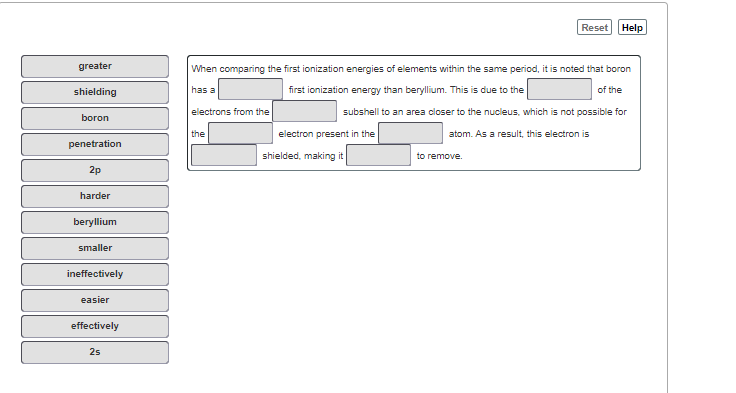
greater
When comparing the first ionization energies of elements within the same period, it is noted that boron
shielding
has a
first ionization energy than beryllium. This is due to the
of the
electrons from the
subshell to an area closer to the nucleus, which is not possible for
boron
the
electron present in the
atom. As a result, this electron is
penetration
shielded, making it
to remove.
2p
harder
beryllium
smaller
ineffectively
easier
effectively
2s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning