Reset Help trigonal bipyramidal tetrahedral linear trigonal planar octahedral AB5 АВЗ АВЗ AB4 AB6 three-dimensional two-dimensional Notation Named Geometry Features Group 1 Group 2 No defined plane Group 1 Trigonal planar Group 3 Group 1 Tetrahedral Group 3 AB5 Group 2 Group 3 AB6 Group 2 Group 3
Reset Help trigonal bipyramidal tetrahedral linear trigonal planar octahedral AB5 АВЗ АВЗ AB4 AB6 three-dimensional two-dimensional Notation Named Geometry Features Group 1 Group 2 No defined plane Group 1 Trigonal planar Group 3 Group 1 Tetrahedral Group 3 AB5 Group 2 Group 3 AB6 Group 2 Group 3
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter8: Advanced Theories Of Covalent Bonding
Section: Chapter Questions
Problem 35E: Can a molecule with an odd number of electrons ever be diamagnetic? Explain why or why not.
Related questions
Question
Use the Model mode in the PhET simulation to go through the various types of structures that can be made by forming and breaking bonds. The coordination around the central atom can range from 0 to 6, but the geometries that are of interest arise from coordination numbers of 2 to 6. You can click the Molecule Geometry checkbox to have the geometry of the currently drawn structure displayed on screen.
After familiarizing yourself with the simulation, complete the following table that matches geometries, coordination models (notations), and structural features.
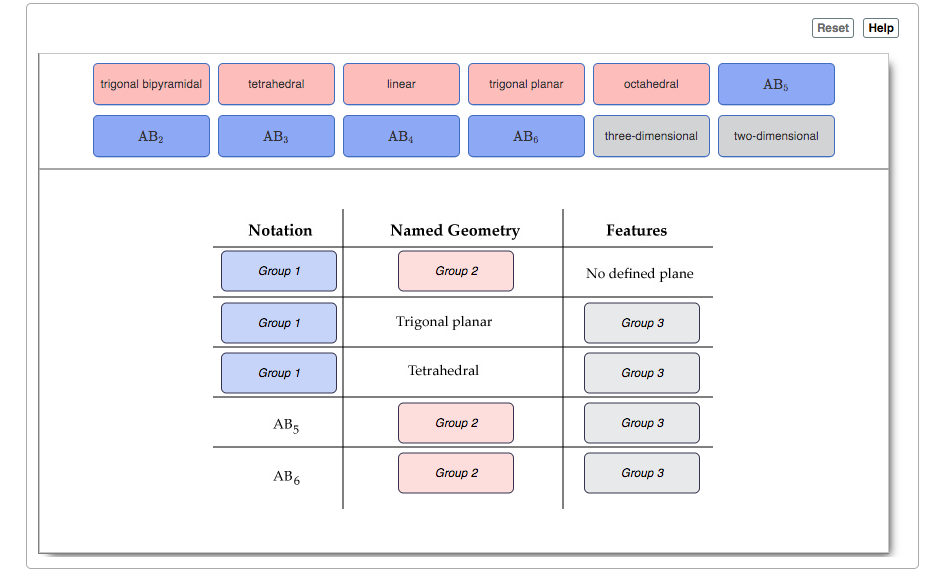
Transcribed Image Text:Reset
Help
trigonal bipyramidal
tetrahedral
linear
trigonal planar
octahedral
AB5
АВЗ
АВЗ
AB4
AB6
three-dimensional
two-dimensional
Notation
Named Geometry
Features
Group 1
Group 2
No defined plane
Group 1
Trigonal planar
Group 3
Group 1
Tetrahedral
Group 3
AB5
Group 2
Group 3
AB6
Group 2
Group 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning