Review: Complete the following table: Element Average Atomic Mass (amu) Chemical Atomic Number of Number of Number of Mass of 1 Symbol Number protons electrons neutrons atom (amu) Ca 20 krypton 1 1
Review: Complete the following table: Element Average Atomic Mass (amu) Chemical Atomic Number of Number of Number of Mass of 1 Symbol Number protons electrons neutrons atom (amu) Ca 20 krypton 1 1
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 8CLE
Related questions
Question
100%
Transcribed Image Text:dres Carrillo - Alchemy Lessons 18 & 19 Worksheet
☆回O
Edit View Insert Format Tools Add-ons Help
Last edit was yesterday at 10:00 AM
三
三,三
11
B IUA
100%
Normal text
Arial
8.
6
3
4
1
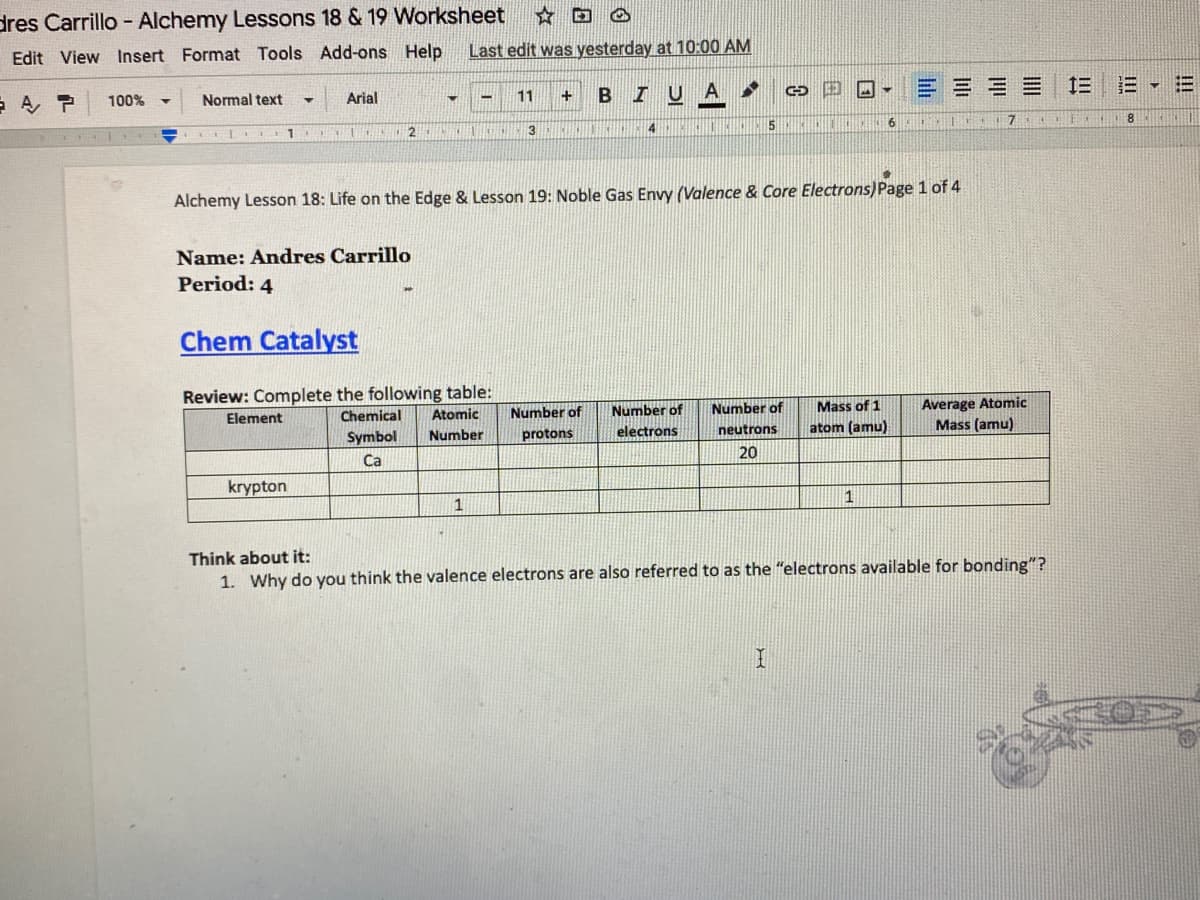
Alchemy Lesson 18: Life on the Edge & Lesson 19: Noble Gas Envy (Valence & Core Electrons)Page 1 of 4
Name: Andres Carrillo
Period: 4
Chem Catalyst
Review: Complete the following table:
Chemical
Average Atomic
Mass (amu)
Number of
Number of
Number of
Mass of 1
Element
Atomic
protons
electrons
neutrons
atom (amu)
Symbol
Number
20
Ca
krypton
1
1
Think about it:
1. Why do you think the valence electrons are also referred to as the "electrons available for bonding"?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning