Review Constants I Penodic Table A beaker with 1.00x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molanity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.40 mL of a 0.460 M HCl solution to the beaker. How much will the pH change? The pK, of acetic acid is 4.740. Express your answer numerically to two decimal places. Use a minus (-) sign if the pH has decreased. > View Available Hint(s) ApH = 4.09 Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining
Review Constants I Penodic Table A beaker with 1.00x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molanity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.40 mL of a 0.460 M HCl solution to the beaker. How much will the pH change? The pK, of acetic acid is 4.740. Express your answer numerically to two decimal places. Use a minus (-) sign if the pH has decreased. > View Available Hint(s) ApH = 4.09 Submit Previous Answers X Incorrect; Try Again; 3 attempts remaining
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter15: Acid–base Equilibria
Section: Chapter Questions
Problem 51P
Related questions
Question
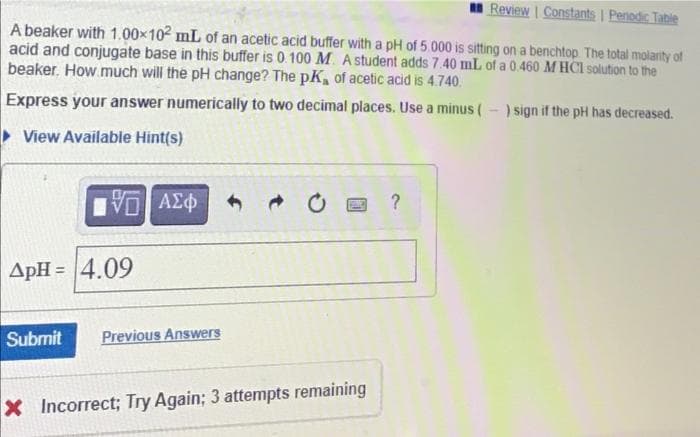
Transcribed Image Text:Review Constants I Penodic Table
A beaker with 1.00x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop The total molarity of
acid and conjugate base in this buffer is 0.100 M. A student adds 7.40 mL of a 0.460 M HCI solution to the
beaker. How much will the pH change? The pK, of acetic acid is 4.740.
Express your answer numerically to two decimal places. Use a minus (-) sign if the pH has decreased.
> View Available Hint(s)
να ΑΣφ
ApH = 4.09
Submit
Previous Answers
X Incorrect; Try Again; 3 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

