Review I Constants I Periodic Table Percent composition refers to the mass percent of each element in a compound: Part A mass of element V mass percent mass of compound X 100% A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: C8H18 For example, the percent composition of water, H2O, is 11.2% hydrogen and 88.8% oxygen. Therefore, a 100-g sample of water contains 11.2 g of hydrogen atoms and 88.8 g of oxygen atoms. Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. View Available Hint(s) The periodic table will be useful when doing this problem. You can access a periodic table by clicking the "Tools" link in the upper right corner of this page. α ΑΣφ ? |80,20 carbon, hydrogen = Previous Answers Submit X Incorrect; Try Again; 3 attempts remaining
Review I Constants I Periodic Table Percent composition refers to the mass percent of each element in a compound: Part A mass of element V mass percent mass of compound X 100% A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: C8H18 For example, the percent composition of water, H2O, is 11.2% hydrogen and 88.8% oxygen. Therefore, a 100-g sample of water contains 11.2 g of hydrogen atoms and 88.8 g of oxygen atoms. Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas. View Available Hint(s) The periodic table will be useful when doing this problem. You can access a periodic table by clicking the "Tools" link in the upper right corner of this page. α ΑΣφ ? |80,20 carbon, hydrogen = Previous Answers Submit X Incorrect; Try Again; 3 attempts remaining
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter8: Chemical Composition
Section: Chapter Questions
Problem 128CP: itamin B12 , cyancobalamin, is essential for human nutrition. Its molecular formula is...
Related questions
Question
100%
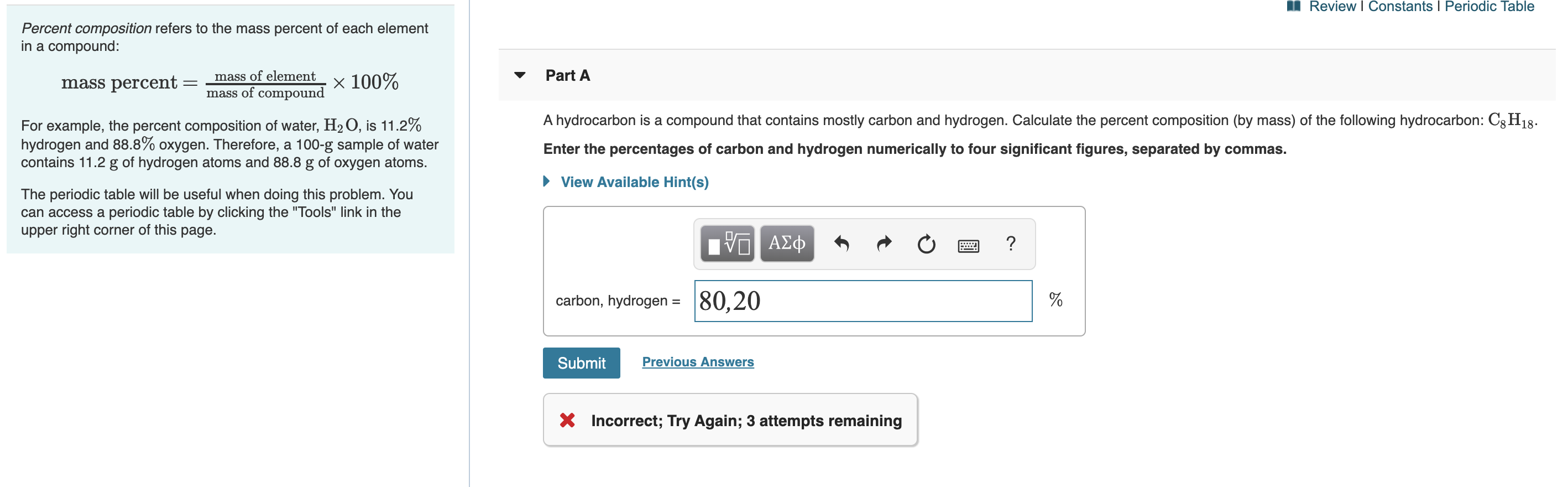
Transcribed Image Text:Review I Constants I Periodic Table
Percent composition refers to the mass percent of each element
in a compound:
Part A
mass of element
V
mass percent
mass of compound X 100%
A hydrocarbon is a compound that contains mostly carbon and hydrogen. Calculate the percent composition (by mass) of the following hydrocarbon: C8H18
For example, the percent composition of water, H2O, is 11.2%
hydrogen and 88.8% oxygen. Therefore, a 100-g sample of water
contains 11.2 g of hydrogen atoms and 88.8 g of oxygen atoms.
Enter the percentages of carbon and hydrogen numerically to four significant figures, separated by commas.
View Available Hint(s)
The periodic table will be useful when doing this problem. You
can access a periodic table by clicking the "Tools" link in the
upper right corner of this page.
α ΑΣφ
?
|80,20
carbon, hydrogen =
Previous Answers
Submit
X Incorrect; Try Again; 3 attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
