Review I Constants I Periodic Table This figure (Figure 1) shows a container that is sealed at the top by a movable piston. Inside the container is an ideal gas at 1.00 atm, 20.0° C, and 1.00 L. This information will apply to the first three parts of this problem Part A What will the pressure inside the container become if the piston is moved to the 2.20 L mark while the temperature of the gas is kept constant? Express your answer with the appropriate units. View Available Hint(s) Value Units P = Submit Part B Figure 1 of 1 The gas sample has now returned to its original state of 1.00 atm, 20.0 °C, and 1.00 L. What will the pressure become if the temperature of the gas is raised to 200.0°C and the piston is not allowed to move? Express your answer with the appropriate units. View Available Hint(s) 2 L piston container ? μΑ F1L Value Units P = -ideal gas sample Submit
Review I Constants I Periodic Table This figure (Figure 1) shows a container that is sealed at the top by a movable piston. Inside the container is an ideal gas at 1.00 atm, 20.0° C, and 1.00 L. This information will apply to the first three parts of this problem Part A What will the pressure inside the container become if the piston is moved to the 2.20 L mark while the temperature of the gas is kept constant? Express your answer with the appropriate units. View Available Hint(s) Value Units P = Submit Part B Figure 1 of 1 The gas sample has now returned to its original state of 1.00 atm, 20.0 °C, and 1.00 L. What will the pressure become if the temperature of the gas is raised to 200.0°C and the piston is not allowed to move? Express your answer with the appropriate units. View Available Hint(s) 2 L piston container ? μΑ F1L Value Units P = -ideal gas sample Submit
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.60PAE: 60 Automakers are always investigating reactions for the generation of gas to inflate air bags, in...
Related questions
Question
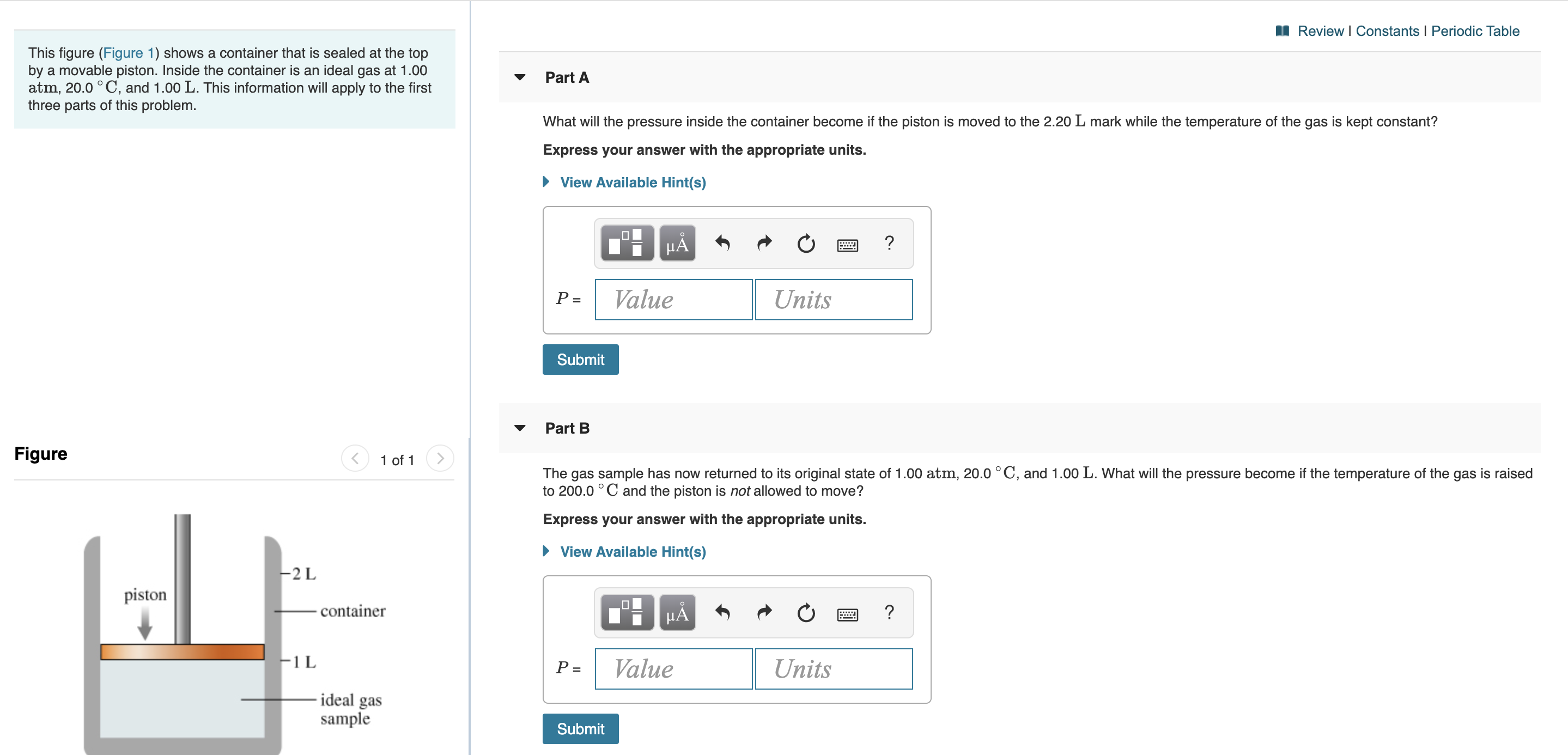
Transcribed Image Text:Review I Constants I Periodic Table
This figure (Figure 1) shows a container that is sealed at the top
by a movable piston. Inside the container is an ideal gas at 1.00
atm, 20.0° C, and 1.00 L. This information will apply to the first
three parts of this problem
Part A
What will the pressure inside the container become if the piston is moved to the 2.20 L mark while the temperature of the gas is kept constant?
Express your answer with the appropriate units.
View Available Hint(s)
Value
Units
P =
Submit
Part B
Figure
1 of 1
The gas sample has now returned to its original state of 1.00 atm, 20.0 °C, and 1.00 L. What will the pressure become if the temperature of the gas is raised
to 200.0°C and the piston is not allowed to move?
Express your answer with the appropriate units.
View Available Hint(s)
2 L
piston
container
?
μΑ
F1L
Value
Units
P =
-ideal gas
sample
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co