I Review I Constants I Perio This figure (Figure 1)shows a container that is sealed at the top by a movable piston. Inside the container is an ideal gas at 1.00 atm, 20.0 °C, and 1.00 L. This information will apply to all parts of this problem A, B, and C. Part B The gas sample has now returned to its original state of 1.00 atm, 20.0 °C and 1.00 L. What will the pressure become if the temperature of the gas is raised to 200.0 °C and the piston is not allowed to move? Express your answer with the appropriate units. • View Available Hint(s) HA ? P = Value Units Submit Figure < 1 of 1 > Part C The gas described in parts A and B has a mass of 1.66 grams. The sample is most likely which monoatomic gas? Type the elemental symbol of the gas below. -2L • View Available Hint(s) piston container symbol = F1 L ideal gas Submit samnle
I Review I Constants I Perio This figure (Figure 1)shows a container that is sealed at the top by a movable piston. Inside the container is an ideal gas at 1.00 atm, 20.0 °C, and 1.00 L. This information will apply to all parts of this problem A, B, and C. Part B The gas sample has now returned to its original state of 1.00 atm, 20.0 °C and 1.00 L. What will the pressure become if the temperature of the gas is raised to 200.0 °C and the piston is not allowed to move? Express your answer with the appropriate units. • View Available Hint(s) HA ? P = Value Units Submit Figure < 1 of 1 > Part C The gas described in parts A and B has a mass of 1.66 grams. The sample is most likely which monoatomic gas? Type the elemental symbol of the gas below. -2L • View Available Hint(s) piston container symbol = F1 L ideal gas Submit samnle
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 4STP
Related questions
Question
Part C only, please.
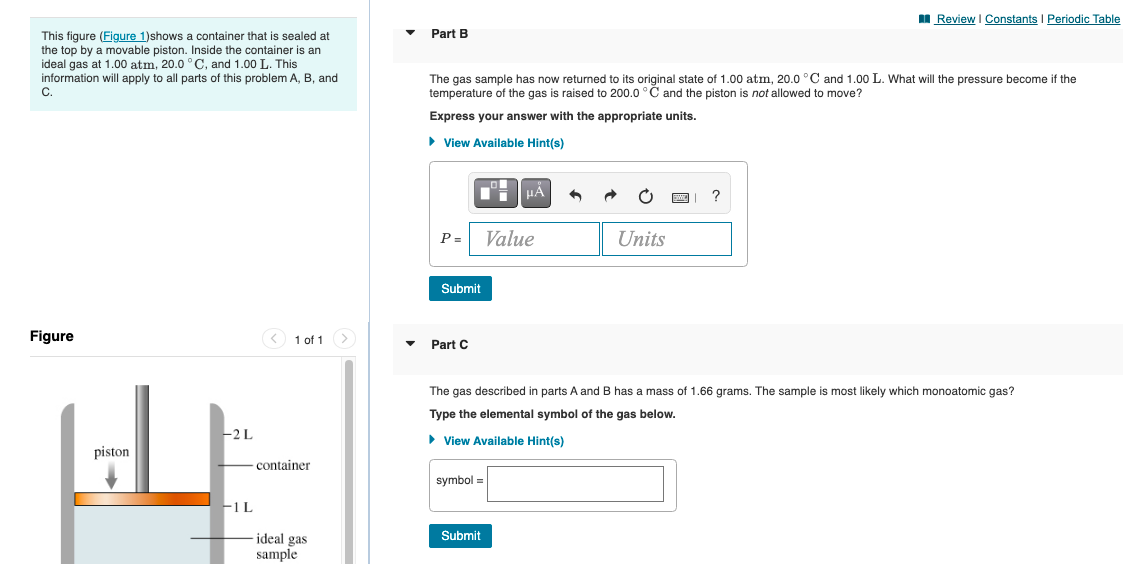
Transcribed Image Text:I Review I Constants I Periodic Table
Part B
This figure (Figure 1)shows a container that is sealed at
the top by a movable piston. Inside the container is an
ideal gas at 1.00 atm, 20.0 ° C, and 1.00 L. This
information will apply to all parts of this problem A, B, and
The
s sample has now returned to its original state of 1.00 atm, 20.0 °C and 1.00 L. What will the pressure become if the
C.
temperature of the gas is raised to 200.0 °C and the piston is not allowed to move?
Express your answer with the appropriate units.
• View Available Hint(s)
HẢ
?
P =
Value
Units
Submit
Figure
< 1 of 1 >
Part C
The gas described in parts A and B has a mass of 1.66 grams. The sample is most likely which monoatomic gas?
Type the elemental symbol of the gas below.
-2L
• View Available Hint(s)
piston
-container
symbol =
F1 L
ideal gas
Submit
sample
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning