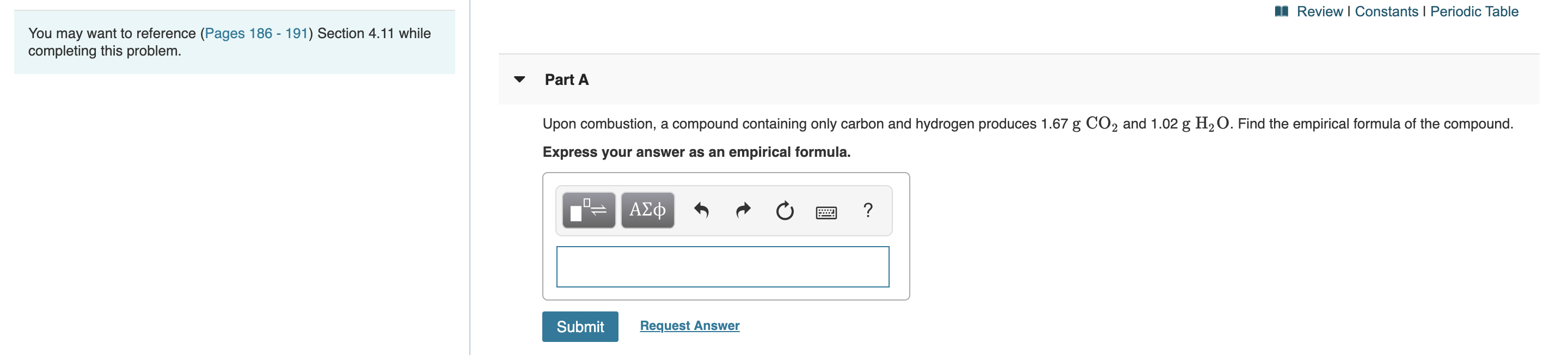
Review I Constants I Periodic Table You may want to reference (Pages 186 - 191) Section 4.11 while completing this problem. Part A Upon combustion, a compound containing only carbon and hydrogen produces 1.67 g CO2 and 1.02 g H2O. Find the empirical formula of the compound. Express your answer as an empirical formula. ΑΣφ ? E Request Answer Submit
Review I Constants I Periodic Table You may want to reference (Pages 186 - 191) Section 4.11 while completing this problem. Part A Upon combustion, a compound containing only carbon and hydrogen produces 1.67 g CO2 and 1.02 g H2O. Find the empirical formula of the compound. Express your answer as an empirical formula. ΑΣφ ? E Request Answer Submit
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 2.DCP
Related questions
Question
Transcribed Image Text:Review I Constants I Periodic Table
You may want to reference (Pages 186 - 191) Section 4.11 while
completing this problem.
Part A
Upon combustion, a compound containing only carbon and hydrogen produces 1.67 g CO2 and 1.02 g H2O. Find the empirical formula of the compound.
Express your answer as an empirical formula.
ΑΣφ
?
E
Request Answer
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning