Part B The probability of finding an electron at a point in an atom is referred to as the probability density (). The spatial distribution of these densities can be derived from the radial wave function R(r) and angular wave function Y(0, 4), then solving the Schrödinger equation for a specific set of quantum numbers. Which of the following statements about nodes and probability density are accurate? Select all that apply. • View Available Hint(s) O The 4f orbitals have three nodes. O The 3p orbitals have two nodes. O The 2s orbital does not have any nodes. O The probability of finding an electron at the center of a d orbital is greater than zero. O The probability of finding an electron at the center of a p orbital is zero.
Part B The probability of finding an electron at a point in an atom is referred to as the probability density (). The spatial distribution of these densities can be derived from the radial wave function R(r) and angular wave function Y(0, 4), then solving the Schrödinger equation for a specific set of quantum numbers. Which of the following statements about nodes and probability density are accurate? Select all that apply. • View Available Hint(s) O The 4f orbitals have three nodes. O The 3p orbitals have two nodes. O The 2s orbital does not have any nodes. O The probability of finding an electron at the center of a d orbital is greater than zero. O The probability of finding an electron at the center of a p orbital is zero.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 48AP: The wave function of an electron in the lowest (that is, ground) state of the hydrogen atom is (r)=(...
Related questions
Question
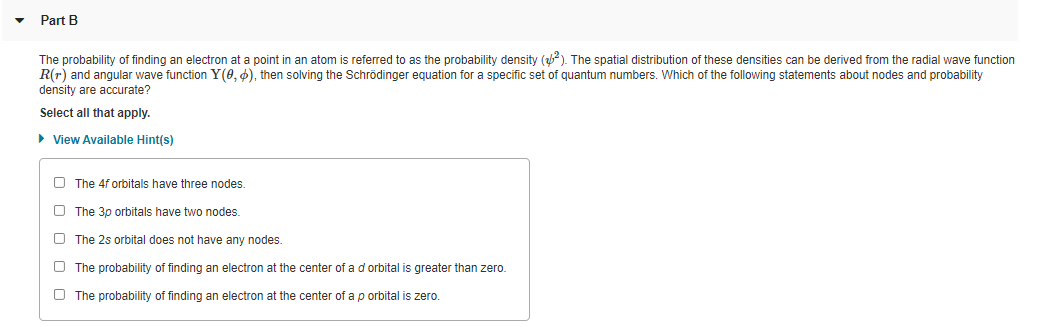
Transcribed Image Text:Part B
The probability of finding an electron at a point in an atom is referred to as the probability density (). The spatial distribution of these densities can be derived from the radial wave function
R(r) and angular wave function Y(0, 6), then solving the Schrödinger equation for a specific set of quantum numbers. Which of the following statements about nodes and probability
density are accurate?
Select all that apply.
• View Available Hint(s)
O The 4f orbitals have three nodes.
O The 3p orbitals have two nodes.
O The 2s orbital does not have any nodes.
O The probability of finding an electron at the center of a d orbital is greater than zero.
O The probability of finding an electron at the center of a p orbital is zero.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning