[Review Topics] [References) Use the References to access important values if needed for this question. Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction: I2(s) + 2Cu*(aq)–→21(aq) + 2Cu²*(ag) Answer: kJ K for this reaction would be than one. Submit Answer Retry Entire Group 9 more group attempts remaining
[Review Topics] [References) Use the References to access important values if needed for this question. Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction: I2(s) + 2Cu*(aq)–→21(aq) + 2Cu²*(ag) Answer: kJ K for this reaction would be than one. Submit Answer Retry Entire Group 9 more group attempts remaining
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 66E
Related questions
Question
![[Review Topics]
[References)
Use the References to access important values if needed for this question.
Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction:
I2(s) + 2Cu*(aq)–→21(aq) + 2Cu²*(ag)
Answer:
kJ
K for this reaction would be
than one.
Submit Answer
Retry Entire Group
9 more group attempts remaining](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F5f21210a-7339-4298-9d4c-227aab5be22c%2Fd50f3c4a-50df-4e19-b748-946ef76b4ea4%2Fpyzo6h.png&w=3840&q=75)
Transcribed Image Text:[Review Topics]
[References)
Use the References to access important values if needed for this question.
Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction:
I2(s) + 2Cu*(aq)–→21(aq) + 2Cu²*(ag)
Answer:
kJ
K for this reaction would be
than one.
Submit Answer
Retry Entire Group
9 more group attempts remaining
Expert Solution
Introduction
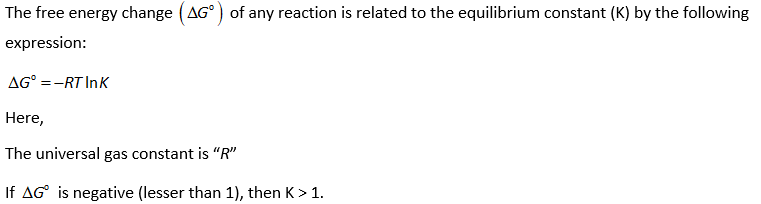
Calculations
The given reaction is as follows:
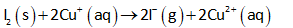
For the given reaction, the half-reduction reaction (at cathode) can be written as follows:
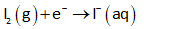
The standard reduction potential for the above reaction is 0.536 V.
The half-reduction reaction (at cathode) can be written as follows:
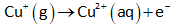
The standard reduction potential for the above reaction is 0.159 V.
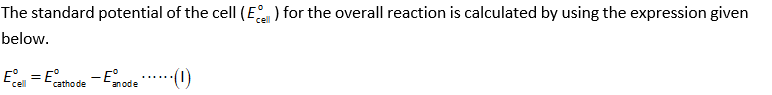
Now, substitute the values in the expression (I).
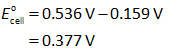
The standard free energy is calculated by using the relation shown below.
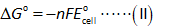
Here,
The number of electrons transfer in the reaction is “n”.
The Faraday’s constant is “F” (96500 C/mol).
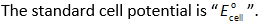

Step by step
Solved in 4 steps with 12 images

Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax